Covidien Receives U.S. Food and Drug Administration Clearance for LigaSure™ Maryland Jaw
BOULDER, Colo.--(BUSINESS WIRE)--Jan. 22, 2014-- Expanding its industry-leading vessel and tissue sealing portfolio, Covidien (NYSE:COV) received U.S. Food and Drug Administration (FDA) 510(k)...
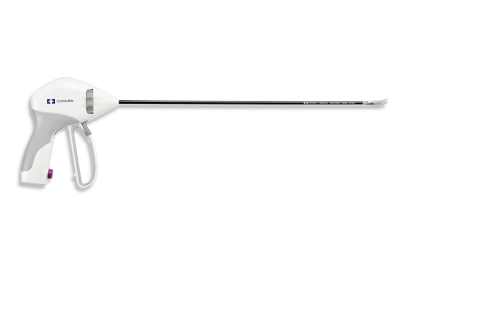
Designed to improve efficiency during laparoscopic and open surgery, the LigaSure Maryland jaw device combines LigaSure’s energy-based vessel sealing technology with the functionality of three common surgical tools: a
“For more than 15 years, surgeons have trusted LigaSure technology for its ability to reduce blood loss,1,2 shorten procedure time1,2 and shorten the length of hospital stay1 compared to sutures,” said
Setting the industry standard, LigaSure technology has been used in more than 8 million vessel sealing procedures worldwide.
LigaSure vessel sealing technology is powered by the ForceTriad™ energy platform, controlled by TissueFectTM sensing technology, which monitors changes in tissue 3,333 times per second and adjusts energy output accordingly to deliver the appropriate amount of energy for the desired tissue effect. LigaSure vessel sealing uses the body’s own collagen and elastin to create a permanent fusion zone. Covidien’s proprietary technology can fuse vessels up to and including 7 mm, lymphatics, tissue bundles and pulmonary vasculature.
The LigaSure Maryland jaw device comes in three lengths.
For more information on LigaSure products, visit: http://surgical.covidien.com/products/vessel-sealing#technology
ABOUT
1 Ding Z, Wable G, Rane A. Use of Ligasure bipolar diathermy system in vaginal hysterectomy. J Obstet Gynaecol. 2005;25(1): 49-51.
2 Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147-151.
Photos/Multimedia Gallery Available: http://www.businesswire.com/multimedia/home/20140122005390/en/
Source:
Covidien plc
John Jordan, 508-452-4891
Manager, Communications
john.jordan@covidien.com
or
Coleman Lannum, CFA, 508-452-4343
Vice President, Investor Relations
cole.lannum@covidien.com
or
Marguerite Copel, 203-821-4720
Vice President, Communications
marguerite.copel@covidien.com
or
Todd Carpenter, 508-452-4363
Senior Director, Investor Relations
todd.carpenter@covidien.com
