National Osteoporosis Foundation Supports New Evidence-Based Care Pathway Designed to Optimize Care for Vertebral Compression Fractures (VCF)
DUBLIN, Sept. 16, 2019 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced that the National Osteoporosis Foundation (NOF) supports the VCF Care...
DUBLIN,
|
|||||
Osteoporosis is a common cause of VCF,2,[3]a debilitating and costly condition. In 2000, there were an estimated 9 million new osteoporotic fractures that came to medical attention, of which 1.4 million occurred in the spine (VCF).4The incidence of VCFs is likely to increase as the nation’s population ages.5VCFs decrease mobility and can also cause severe pain, often leading to increased use of opioid pain medications and decreased quality of life.2, 3 Untreated VCFs can lead to a downward spiral that progresses from back pain, to deformed spines, reduced physical function, limited mobility and, finally, increased mortality.6-10Direct costs of VCFs in the United States are estimated to be
The NOF believes that educating the healthcare community on appropriate patient care and working towards national standards are critical for advancing care of VCF patients. NOF has recently recorded and featured a discussion of the VCF Care Pathway on the NOF Bone Talk podcast.
“Our goal in working to integrate the VCF Care Pathway algorithm into commercially-available electronic medical records is to standardize and optimize care for VCF patients regardless of where in the care continuum they present.” said Joshua Hirsch, M.D., vice chair, Procedural Services and chief of the neurointerventional spine service at
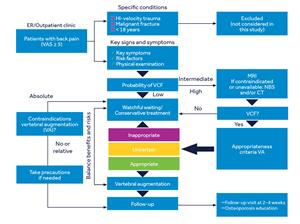
A lack of consensus within the medical community and different levels of familiarity with the literature have made it challenging for healthcare providers to identify those VCF patients who may benefit from vertebral augmentation (VA) and those who could be appropriately managed with conservative care. The VCF Care Pathway is designed to systematically address this challenge.
The VCF Care Pathway was developed using the RAND™/UCLA Appropriateness Method (RAM), a highly-structured approach for developing patient-specific recommendations that combines best available clinical evidence with the collective judgments of a multispecialty panel of experts. To develop the VCF Care Pathway, the panel considered 576 potential clinical scenarios.
“The NOF supports the development and implementation of the VCF Care Pathway,” saidElizabeth Thompson, CEO of
“As a leader in medical technology, Medtronic knows that VA procedures have provided clinical benefits to VCF patients,” said Jeff Cambra, vice president and general manager of the Pain Therapies Interventional business, which is part of the
About the RAND™/UCLA Appropriateness Method and Development of the VCF Care Pathway
The RAND™/UCLA Appropriateness Method (RAM), is a highly structured approach for developing patient-specific recommendations that combines best available clinical evidence with the collective judgments of a multispecialty panel of experts (neurosurgery, interventional neurology, radiology, pain medicine, and orthopedic surgery).To develop the VCF Care Pathway, a 12-member panel of experts reviewed and evaluated the 83 studies related to the management of VCF. The VCF Clinical Care Pathway supports early diagnosis and appropriate treatment of VCF regardless of the patient’s entry point into the healthcare system by identifying patients most likely to benefit from non-surgical management (NSM) as well as those for whom vertebral augmentation (VA) may be most appropriate. The panel members reviewed 20 signs and symptoms associated with VCF and considered the relevance of five procedures, comprising 576 clinical scenarios.
About Balloon Kyphoplasty
Balloon Kyphoplasty is a minimally invasive procedure for the treatment of pathological fractures of the vertebral body due to osteoporosis, cancer, or benign lesion. The complication rate with BKP has been demonstrated to be low. There are risks associated with the procedure (e.g., cement extravasation), including serious complications, and though rare, some of which may be fatal. Risks of acrylic bone cements include cement leakage, which may cause tissue damage, nerve or circulatory problems, and other serious adverse events, such as: cardiac arrest, cerebrovascular accident, myocardial infarction, pulmonary embolism, or cardiac embolism. For complete information regarding indications for use, contraindications, warnings, precautions, adverse events, and methods of use, please reference the devices’ Instructions for Use included with the product.
About the
Established in 1984, the
About Medtronic
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the
*The VCF Pathway published in Spine Journalin 20181was supported by a grant from Medtronic. However, Medtronic was not involved in the design or execution of the project, nor the preparation and review of this manuscript. Names of panel members were not disclosed to the sponsor, and panel members were not informed about the identity of the sponsor before submission of the manuscript. Limitation: Double-blinded doesn’t mitigate that the panel process was subjective input. It included strict adherence by the multidisciplinary experts to adopt the RAND™ methodology process, based on published clinical evidence and expertise.
VCF Care Pathway image is reprinted fromSpine Journal 2018, Hirsch JA, Beall DP, Chambers MR, et al. Management of vertebral fragility fractures: aclinical care pathway developed by a multispecialty panel using the RAND/UCLA Appropriateness Method.Nov;18(11):2152–2161 Copyright 2018, with permission from Elsevier.
1Hirsch JA, et al. Spine J. 2018;18(11):2152–2161.
2Evans AJ, et al. Radiology. 2003;226(2):366–372.
3Ross PD. Am J Med. 1997;103(2A):30S–43S.
4IOF. https://www.iofbonehealth.org/breaking-spine-report-2010. Accessed July 25, 2016.
5Burg R, et al. J Bone Miner Res. 2007;22:465–75.
6Brunton S, et al. J Fam Pract. 2005;54(9):781–788.
7Vedantam R. Am J Clin Med. 2009;6(4):14–18.
8Gold DT. Bone. 1996;18(3 Suppl):185S–189S.
9Ross PD. Am J Med. 1997;103(2A):30S–43S.
10Silverman SL. Bone. 1992;13 Suppl 2:S27–S31.
Victor Rocha
Public Relations
+1-901-399-2401
Ryan Weispfenning
Investor Relations
+1-763-505-4626
Attachment
Source: Medtronic plc