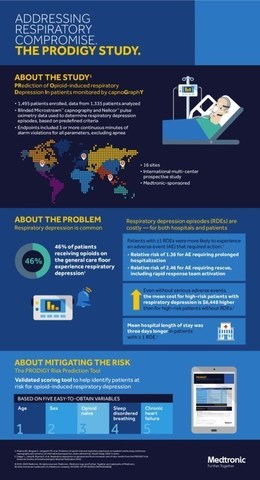
The study, which analyzed 1,335 patients across 16 sites in
"PRODIGY data confirms that respiratory depression in patients receiving parenteral opioids occur frequently and are potentially unknown to hospital healthcare providers," said
Currently, there are no universally accepted guidelines to direct effective and safe assessment and monitoring practices for patients receiving in-hospital opioid analgesia.2 In addition to providing insight into the rate of respiratory depression, a key objective of PRODIGY was to develop and validate an accurate and easy-to-use risk assessment scoring tool. The PRODIGY Risk Score uses risk factors including: age > 60 years, male gender, opioid naïvety, sleep disorders and chronic heart disease for respiratory depression events risk prediction.1
"The PRODIGY Risk Score has acceptable accuracy for risk stratification using several robust methods of internal validation, addressing significant gaps in preventing this common and potentially deadly condition," said
The PRODIGY study is the largest known study using continuous capnography and pulse oximetry data on surgical and medical patients collected by Medtronic Microstream™ and Nellcor™ monitoring technology. The study design used an innovative mechanism of data collection whereby bedside providers were blinded to continuous monitoring systems and all alarms were also silenced. All patients experiencing respiratory depression were reviewed and confirmed by an independent clinical event committee of physicians with expertise in perioperative respiratory medicine.
For additional information about monitoring for respiratory compromise with capnography in the post-operative and general care settings, please visit: medtronic.com/prodigy
About Respiratory Compromise
Respiratory compromise is a potentially life-threatening, progressive condition negatively impacting a person's ability to breathe adequately to maintain oxygenation and carbon dioxide removal. Patients with respiratory depression may experience shallow, slow or no breathing after opioid administration which undetected can lead to cardiopulmonary arrest and death.3 This condition is rapidly becoming the third-most costly hospital inpatient expense in the
About Medtronic
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the
- Khanna AK, Bergese S, Jungquist CR, Morimatsu H, Uezono S, Lee S, et al. Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg. 2020; In press.
- Khanna AK, Overdyk FJ, Greening C, Di Stefano P, Buhre WF. Respiratory depression in low acuity hospital settings – seeking answers from the PRODIGY trial. J of
Crit Care 2018, https://www.jccjournal.org/article/S0883-9441(18)30217-X/fulltext. AccessedJune 18, 2018 . - Morris TA, Gay PC,
MacIntyre NR , et al. Respiratory compromise as a new paradigm for the care of vulnerable hospitalized patients. Respir Care. 2017 Apr;62(4):497-512. doi: 10.4187/respcare.05021. - Wier LM, Henke R, Friedman B. Diagnostic groups with rapidly iCosts, by payer, 2001-2007: statistical brief #91.
Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville MD2010. - Belcher AW, Khanna AK, Leung S, et al. Long-acting patient-controlled opioids are not associated with more postoperative hypoxemia than short-acting patient-controlled opioids after noncardiac surgery: a cohort analysis. Anesth Analg. 2016;123(6):1471-9.
- Khanna AK, Sessler DI, Sun Z, et al. Using the STOP-BANG questionnaire to predict hypoxaemia in patients recovering from noncardiac surgery: a prospective cohort analysis. Brit J Anaesth. 2016;116(5):632-40.
- Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg. 2015;121(3):709-15.
|
Contacts: |
|
|
|
|
|
Public Relations |
Investor Relations |
|
+1-508-452-4891 |
+1-763-505-4626 |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/medtronic-study-finds-opioid-induced-respiratory-depression-in-the-hospital-more-common-and-costly-301124579.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/medtronic-study-finds-opioid-induced-respiratory-depression-in-the-hospital-more-common-and-costly-301124579.html
SOURCE