Medtronic CoreValve/Evolut™ demonstrates best durability
CRT 2023 Late-Breaking Data: CoreValve/Evolut™ platform demonstrates significantly lower bioprosthetic valve dysfunction compared to surgery at five years
Medtronic today announced five-year bioprosthetic valve dysfunction (BVD) data for the CoreValve/EvolutTM platform, the first and only transcatheter aortic valve replacement (TAVR) platform to show significantly better valve performance and durability compared to surgical aortic valve replacement (SAVR) at five years. Data, presented by Dr. Steven Yakubov, in a late-breaking clinical trial session at the 2023 annual meeting of Cardiovascular Research Technologies (CRT 2023), build upon the excellent results of the structural valve deterioration (SVD) analysis recently published in JAMA Cardiology.
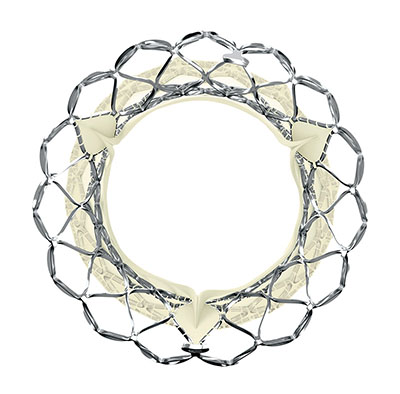
The CoreValve and next generation Evolut TAVR systems are used in an aortic valve replacement procedure that is less invasive than traditional open-heart surgery for patients with severe aortic stenosis.
“These results add yet another proof point demonstrating the durability benefits of TAVR compared to surgery when it comes to bioprosthetic valve dysfunction – a complication that can impact the durability and performance of a critical valve replacement,” said Steven J. Yakubov, M.D., System Chief of Structural Heart Disease at the Ohio Health, Riverside Methodist Hospital. “With valve durability becoming more critical as TAVR expands to younger, healthier patients, this analysis underscores the long-term promise of this minimally invasive approach and should be taken into consideration during initial valve selection.”
The study retrospectively analyzed 1,128 TAVR and 971 surgery patients from the CoreValve U.S. High Risk and SURTAVI randomized trials. The pooled analysis of the CoreValve U.S. Pivotal and SURTAVI Trials evaluated valve performance and durability of surgical and transcatheter valves by incidence of overall BVD which includes structural valve deterioration (SVD), non-structural valve dysfunction (NSVD), thrombosis, and endocarditis. This is the first comprehensive analysis to support clinical criteria for valve performance and durability using all four components of BVD and its association with clinical outcomes.
“These extraordinary results reinforce the hemodynamic durability and valve performance of the CoreValve/Evolut system and demonstrate again that Evolut is a safe and effective alternative to surgery,” said Nina Goodheart, senior vice president and president of the Structural Heart & Aortic business, which is part of the Cardiovascular portfolio at Medtronic. “As a pioneer and leader in the early treatment of aortic stenosis, Medtronic strives to continually improve our CoreValve/Evolut platform to ensure it is meeting the needs of the physicians and the growing patient population that will benefit from this type of procedure.”
In the study, CoreValve/EvolutTM had a significantly lower BVD cumulative incidence rate compared to surgery (7.8% vs. 14.2%; p<0.001). Five-year rates of structural valve deterioration and non-structural valve dysfunction were significantly lower after TAVR compared to surgery [SVD-TAVR 2.2% vs SAVR 4.4%; p 0.004, NSVD-TAVR 4.3% vs SAVR 8.8%; p<0.001]. TAVR also demonstrated three times lower severe prosthesis-patient mismatch (PPM) (3.7% vs. 11.8%; p<0.001) at 30-days/discharge compared to surgery, which occurs when the valve is too small for the patient's body size. There were no significant differences in rates of thrombosis (0.3% TAVR vs 0.2% SAVR, p=0.80) or endocarditis (1.1% TAVR vs. 1.3% SAVR, p=0.68).
Patients in both groups who developed BVD through 5 years were at about 1.5-fold greater risk of death or hospitalization for valve-related disease or heart failure as patients who did not develop BVD.
The CoreValve U.S. Pivotal High-Risk Trial was a prospective, randomized, multicenter, noninferiority study that compared the safety and efficacy of the Medtronic CoreValve system to SAVR in patients with symptomatic severe aortic stenosis at increased surgical risk. The SURTAVI Trial was a prospective, randomized, multicenter, noninferiority study to assess the safety and efficacy of the Medtronic TAVR system to SAVR in patients with symptomatic severe aortic stenosis at intermediate surgical risk.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Contacts:
Ben Petok
Public Relations
+1-612-297-0501
Ryan Weispfenning
Investor Relations
+1-763-505-4626
