Medtronic real-world evidence demonstrates consistent results of the MiniMed™ 780G regardless of demographic or cultural differences
Data shows the successful performance of the AID algorithm under substantial changes to daily routines and lifestyle, including during Ramadan.
Medtronic plc (NYSE:MDT), a global leader in medical technology, presented multi-continent real-world data demonstrating that use of the MiniMed™ 780G system allows people living with diabetes to meet or exceed internationally recommended targets regardless of region of the world. One of the barriers to automated insulin delivery (AID) adoption and reimbursement in developing and middle-income countries has been the lack of data supporting AID performance and cost effectiveness in these regions due to large disparities in ethnic, cultural and regional representation in clinical and real-world studies on the use and outcomes of technology in type 1 diabetes (T1D). The successful results displayed by this data support calls to provide automated insulin delivery systems to anyone who desires better outcomes and especially among under-served populations. These data sets were presented today at the European Association for the Study of Diabetes (EASD) 59th Annual Meeting in Hamburg, Germany.
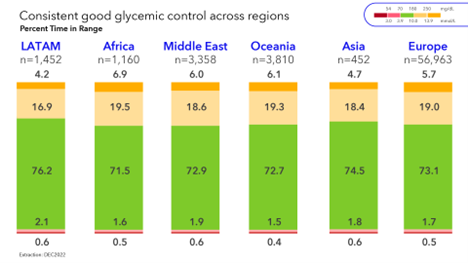
Analysis1 was performed on real-world data of over 67,000 users of the MiniMed™ 780G system users in Europe, Middle East, Africa, Latin America, Oceania, and Asia. The analysis demonstrated that average glycemic control exceeds internationally recommended targets (ADA guidelines recommend 70% time in range between 70-180 mg/dL)2,3 and that the use of recommended settings of the MiniMed™ 780G system further improves glycemic outcomes and narrows the differences across regions. This suggests that the MiniMed™ 780G system nearly equalizes performance across disparate regions of the world.
Providing a solution for Muslims living with diabetes who observe Ramadan
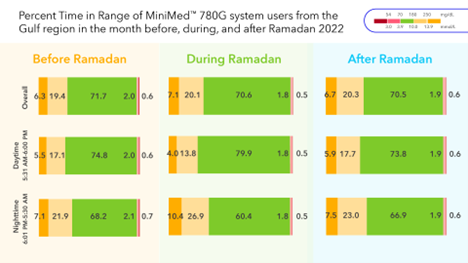
Fasting during the month of Ramadan carries a high risk of complications for people living with type 1 diabetes. These complications include hypoglycemia, hyperglycemia, dehydration, and diabetic ketoacidosis, particularly during the fasting hours (from Fajr to Maghrib prayer). Even though people with diabetes are exempted, many choose to fast. A separate analysis of real-world data shows that people in the Gulf region using the MiniMed™ 780G system over the Ramadan holiday experienced consistent overall time in range, time below range, and mean sugar glucose before, during, and after Ramadan.4 Interestingly, there was no increased risk of hypoglycemia during the Ramadan fasting hours (5:31 a.m. – 6:00 p.m.), despite the presumed risk of fasting for people living with diabetes.
Considering that Ramadan encompasses substantial lifestyle modifications, such as alterations in dietary habits, exercise routines, and sleep patterns, this evidence highlights how the MiniMed™ 780G advanced hybrid closed-loop system, with its SmartGuard™ algorithm, not only performs consistently across different demographics and cultures, but it is proven to adapt to other scenarios involving lifestyle changes of people living with type 1 diabetes.
“As AID systems have taken a foothold among western countries, we’ve seen the growing success of outcomes from algorithms, such as SmartGuard™ technology, to reduce the burden of living with diabetes. That said, there continues to be gaps in access among under-served populations given false perceptions about the impacts of cultural differences to the ultimate success of patients. With the growing body of data, we’re proving that the ideal user of the MiniMed™ 780G system is anyone who is living with diabetes, regardless of region, religion, or cultural differences,” said Federico Gavioli, Senior Vice President, Medtronic Diabetes, EMEA & Americas.
Challenging Technology to Consistently Achieve Time in Tight Range
As the clinical and real-world evidence for the MiniMed™ 780G system continues to build, the system is demonstrating its ability to support individuals achieving even greater outcomes. People who live with diabetes are encouraged to achieve >70% TIR (70-180 mg/dl). The recently introduced time in tight range (TITR), which prior to the success of algorithms like the MiniMed™ 780G system was considered an aggressive target for diabetes management, lowers the threshold for TIR to 70-140 mg/dl. In an oral presentation today, real-world evidence showed that users of the MiniMed™ 780G system on average reach a TITR of 48.9%, and even up to 56.1% when recommended settings are used.5 Previous evidence has indicated the importance of using these recommended settings for achieving a high TIR, but here it is shown that it is even more crucial to consistently adhere to recommended settings if the objective is to achieve a high TITR.
1 Van den Heuvel T, Arrieta A, Castaneda J, Vigersky R, Cohen O. Consistent performance of the MiniMed 780G system across continents: a world-wide real-world analysis. 59th EASD Annual Meeting of the European Association for the Study of Diabetes.
2 CGM & Time in Range. American Diabetes Association. Available at: https://diabetes.org/tools-support/devices-technology/cgm-time-in-range. Accessed June 19, 2023.
3 American Diabetes Association (2019). Standards of medical care in diabetes—2019. Diabetes Care, 42(Suppl 1): S61-S70.
4 M. Al-Sofiani M, Al-Guwaihis A, Al-Shaikh A, Adjene A, Al-Amuddin N, Mohannadi D, Arrieta A, Castaneda J, Chaar W, Van den Heuvel T, Cohen O. The MiniMed 780G system adapts to substantial changes in daily routine: lessons from real-world users during Ramadan. 59th EASD Annual Meeting of the European Association for the Study of Diabetes.
5 “The use of optimal system settings in real-world MiniMed 780G system users has a large impact on increasing the time in tight glucose range.” Oral presentation at 59th EASD Annual Meeting of the European Association for the Study of Diabetes by Javier Castañeda, Senior Statistics Manager, Medtronic Diabetes
About the Diabetes Business at Medtronic (www.medtronicdiabetes.com)
Medtronic Diabetes is on a mission to alleviate the burden of diabetes by empowering individuals to live life on their terms, with the most advanced diabetes technology and always-on support when and how they need it. We've pioneered first-of-its-kind innovations for over 40 years and are committed to designing the future of diabetes management through next-generation sensors (CGM), intelligent dosing systems, and the power of data science and AI while always putting the customer experience at the forefront.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
