Medtronic highlights new clinical data demonstrating benefits of spinal cord stimulation in two late-breaking presentations at the North American Neuromodulation Society (NANS) Annual Meeting 2024
86% of subjects preferred closed-loop SCS in a multicenter, randomized, crossover, single blind study of Medtronic’s investigational closed-loop SCS system at one month
24-month European RCT data demonstrates long-term superiority of DTM™ SCS compared with conventional medical management (CMM) for indicated chronic back pain patients not eligible for spine surgery
Medtronic, a global leader in healthcare technology, today announced new data from two clinical trials demonstrating the benefits of its spinal cord stimulation (SCS) technologies in patients with chronic low back pain (CLBP) and leg pain. New data from both studies were accepted as late-breaking presentations at the North American Neuromodulation Society (NANS) annual meeting in Las Vegas.
Medtronic study meets primary objective by effectively reducing overstimulation
Medtronic’s data presentation, “Improvements in Health-Related Quality of Life in Chronic Back/Leg Pain Patients with Closed-loop SCS,” features one and three-month outcomes from its Closed Loop-SCS study in Australia, which is evaluating the long-term performance of the closed-loop system and overall patient experience with Medtronic’s investigational, next-generation, rechargeable spinal cord stimulator. This system is approved in Europe and Japan but is not approved for sale or distribution in the United States and Australia.
Key results include:
- The study met its primary objective, with 89% of subjects reporting a significant reduction in overstimulation relative to open loop (n=28; p<0.001), and 86% reporting a preference for closed-loop during blinded testing at one month.
- Clinically meaningful improvements were observed in pain, physical function, and quality of life at three months with closed-loop SCS. Long-term follow-up for pain and related outcomes had a single-arm study design.
- The study met its secondary objective, with 86% of subjects (n=51) reporting 50% or more reduction in overall back and leg pain at three months.
- 100% of subjects reported satisfaction with closed-loop SCS therapy at three months.
- At three months, over 80% of subjects (n=54) were able to achieve their stated activity goals for daily living with SCS therapy without fear of pain or therapy side effects.
- Although the study did not include a weaning protocol, 35% of subjects (n=37) on opioids at baseline reduced/stopped use at three months, and morphine milligram equivalent reduced from 73.5 to 54.0.
“These early promising results suggest that closed-loop SCS may offer real benefits over fixed-output stimulation,” said A/Prof Marc Russo, Director of Hunter Pain Specialists in Newcastle, Australia and lead investigator. “Could closed-loop SCS eventually become the standard of care? More data is needed, but what we are seeing thus far is encouraging.”
The prospective study is being conducted across seven sites in Australia and includes two parts: in-clinic testing for primary endpoint assessment with a randomized, cross-over, single-blind design at one month, and long-term follow-up for pain outcomes at 3-, 6-, 12-, 18-, and 24-months post device activation. Pain outcomes were evaluated in a single-arm study.
24-month European RCT data demonstrates long-term superiority of DTM™ SCS compared with conventional medical management (CMM) for indicated chronic back pain patients who are ineligible for spine surgery
Medtronic’s data presentation, “24 Month outcomes of Pain Relief and QoL from EU RCT comparing DTM to CMM in patients with no prior history of spine surgery,” presents two-year outcomes comparing Medtronic’s proprietary DTM™ SCS waveform to conventional medical management (CMM).
Key study outcomes include:
- Superior back pain responder rate for DTM™ SCS compared with CMM in patients with chronic low back pain at 12, 18, and 24 months (see figure below).
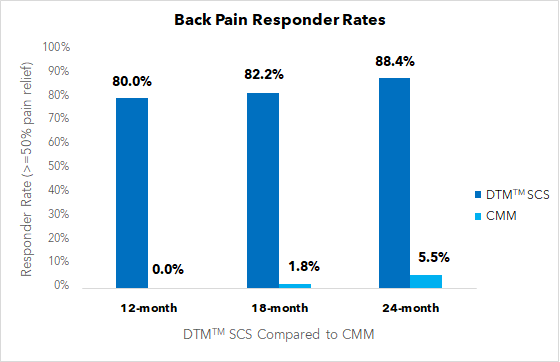
- 77% reduction in chronic low back pain at 24 months for patients using DTM™ SCS.
- 93% leg pain responder rate (subjects with ≥50% leg pain relief) at 24 months for patients using DTM SCS.
- Significant reduction in the extent of disability with DTM™ SCS as measured by the Oswestry Disability Index (ODI): >26 point average ODI reduction at 24 months for patients using DTM SCS.
- 95% of patients were satisfied or very satisfied with DTM™ SCS treatment at 24 months.
“As the first DTM™ SCS RCT in Europe, these results provide additional evidence of the clinical and quality-of-life benefits that DTM™ SCS offers this patient population, which has few available treatment options,” said Dr. Jan Willem Kallewaard, MD, PhD, anesthesiologist at Rijnstate, Elst, The Netherlands, and the study’s lead investigator.
This post-market on-label study is the first EU-based RCT demonstrating long-term benefits of DTM™ SCS for indicated chronic back pain patients ineligible for spinal surgery. The trial was conducted at 12 sites in four European countries and is compared with optimized conventional medical management (CMM). Results demonstrate DTM SCS advantages in pain reduction compared with CCM in indicated chronic back pain patients who are ineligible for spine surgery, including superior responder rates (>= 50% CLBP relief) through 24 months.
“Insights gained today from new clinical evidence pave the way toward the revolutionary therapies of tomorrow, and Medtronic is proud to be leading the way at NANS 2024 with 26 accepted clinical abstracts,” said Paolo Di Vincenzo, president of the Neuromodulation business, which is part of the Neuroscience Portfolio at Medtronic. “We are committed to advancing the field of neuromodulation through rigorous scientific research in order to deliver on our Mission to alleviate pain, restore health and extend life to patients around the world.”
A complete list of Medtronic’s clinical abstracts and presentations at NANS 2024 is available online at Medtronic.com/PainEvents. At NANS, Medtronic is also highlighting the recently FDA approved Percept™ RC neurostimulator with exclusive BrainSense™ technology.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com, and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
