Detecting the (almost) undetectable
FDA clears GI Genius™ intelligent endoscopy module, the first device to use artificial intelligence to find potentially precancerous polyps
Colonoscopies are a critical tool in the fight against colon cancer.
But what if that doctor performing the procedure had access to an extra set of ‘eyes’ trained to find hard-to-spot polyps?
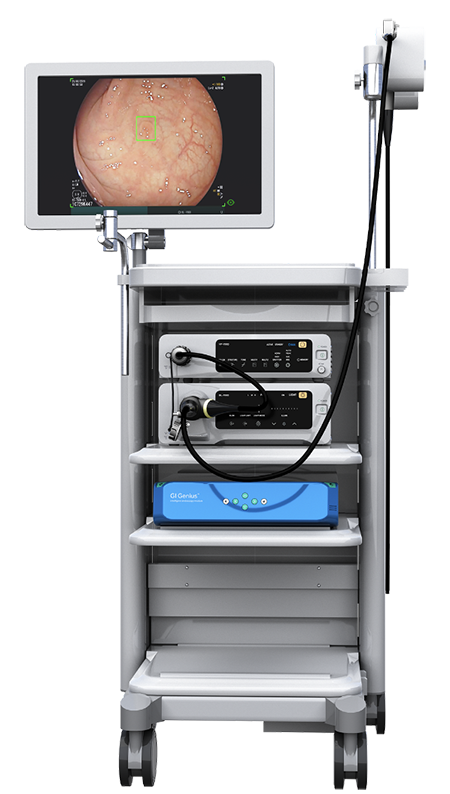
Recently, the Food and Drug Administration (FDA) approved Medtronic’s GI Genius™ intelligent endoscopy module, the only commercially available computer-aided detection system using artificial intelligence (AI) to identify colon polyps during a colonoscopy.
The module promises to be a powerful new tool in the fight against colorectal cancer, which is the third most common form of cancer globally.

"Medtronic is committed to helping prevent colorectal cancer and improving patient outcomes with disruptive technologies that aid screening, increase patient compliance, and improve treatment," says Giovanni Di Napoli, president of the Gastrointestinal business at Medtronic. “By introducing AI technology into the colonoscopy market, we anticipate improving colonoscopy detection rates and reducing variability in patient outcomes"
How AI can help beat colorectal cancer
Through the power of AI, the GI Genius™ system scans every visual frame taken during a colonoscopy procedure in milliseconds and alerts physicians to the presence of lesions — including small, flat polyps that can easily go undetected by the human eye.
In other words, it helps detect the undetected.
"Colonoscopies allow highly skilled gastroenterologists to identify polyps and lesions that might develop into cancer,” said Michael Sapienza, chief executive officer of the Colorectal Cancer Alliance. “With the GI Genius™ system, we can tap into the potential of artificial intelligence approaches to help increase detection rates. This important new development helps us in our mission to detect colon cancer early and to improve patient outcomes.”
Medtronic sought FDA clearance for the GI Genius™ system under what the agency calls its De Novo pathway, which is a process designed for novel technologies requiring applicants to submit information about the safety and effectiveness of their devices as supported by clinical data.
And what the data shows is remarkable — the system delivers a 14% increase in identifying hard-to-detect precancerous lesions. This can assist physicians in locating abnormalities, ultimately reducing the risk of colorectal cancer.
“Medtronic GI Genius™ module is the only commercially available system that is truly plug and play, in that it can integrate with most available endoscopy processors,” said Dr. Mark Ellrichmann, head of Interdisciplinary Endoscopy at University Hospital Schleswig-Holstein in Germany. “AI-assisted colonoscopy will become the gold standard of colonoscopy for detection and differentiation of polyps. From my point of view, it can significantly improve the quality performance of colonoscopy.”
How Medtronic devices are getting ‘smarter’
Artificial intelligence plays a significant role in our daily lives. It does everything from running spellcheck on your email to monitoring traffic. The technology is also driving exciting advances within the medical technology industry.
By creating algorithms capable of analyzing a tremendous amount of data, Medtronic medical devices can be ‘trained’ to identify specific conditions within the human body.
For example, the software in the GI Genius™ module contains a dataset of more than 13 million polyp images of various shapes and sizes. And, as the dataset grows, the GI Genius™ intelligent endoscopy module continues to ‘learn’ and become even ‘smarter.’
“This AI never gets tired or is distracted, helping the physician to find the pathologies and make diagnoses much more quickly,” says Di Napoli
Clinical and Economic Evidence Driving Patient-Centered Care
Read How: Meaningful Innovation
L001-050421
Important Safety Information
Indication
The GI Genius System is a computer-assisted reading tool designed to aid endoscopists in detecting colonic mucosal lesions (such as polyps and adenomas) in real time during standard whitelight endoscopy examinations of patients undergoing screening and surveillance endoscopic mucosal evaluations. The GI Genius computer-assisted detection device is limited for use with standard white-light endoscopy imaging only. This device is not intended to replace clinical decision making.
Danger: Do not use this system for any purpose other than its intended use.
Danger: The system does not perform any diagnosis.
Caution: The sale, distribution, and the use of the GI Genius are restricted to prescription use in accordance with 21 CFR 801.109.
Caution: The device is not intended to be used as a stand-alone diagnostic device.
Caution: The device is not intended to characterize lesions in a manner that would potentially replace biopsy sampling.
Caution: The device is not intended to replace clinical decision making. Caution: The device is not intended to be used with equipment that was not tested against during validation activities. Caution: The device has not been studied in patients with Inflammatory Bowel Disease (IBD), history of CRC, or previous colonic resection. The device performance may be negatively impacted by mucosal irregularities such as background inflammation from certain underlying disease.
Caution: Users should be aware, when using automated systems such as the GI Genius that provide assistance in identifying suspicious lesions, that a high level of reliance may be placed on the system output, potentially leading to user complacency and a bias towards accepting the system output results, rather than using the device as intended. The device is intended to provide adjunctive information to the endoscopist who performs endoscopic evaluations with an expected high level of clinical expertise. Placing too much reliance on the system in this way may lead to an unacceptably low level of procedural oversight ('situational awareness'), sub-par evaluation of the patient, and eventual endoscopist skill degradation, following which retraining may be necessary.
Danger: Using incompatible equipment can result in patient injury or equipment damage and makes it impossible to obtain the expected functionality.
Risks
Electrical Risks Danger
Strictly observe the following precautions. Failure to do so may place the patient and medical personnel in danger of electric shock. Keep fluids away from the system. If fluids are spilled on or into the system, immediately stop any operation of the System and contact Support (see Section 14).
Danger: Do not use the System when not properly closed. Do not touch electrical contacts inside any component of the System.
Fire or Explosion Risks Danger
In order to prevent fire and explosion, do not expose the system where:
- there is a high concentration of oxygen
- air contains oxidizing agents (e.g. N2O)
- air contains any flammable gases
- there are flammable liquids nearby
Interferences
Warning: System may interfere with other equipment.
Caution: Electromagnetic interference may occur to this instrument when it is placed near equipment marked with the following symbol or other portable and mobile RF communications equipment such as cellular phones. If radio interference occurs, mitigation measures may be necessary, such as reorienting or relocating this instrument or shielding the location. Do not use GI Genius system and its cables closer than 30 cm (12 inches) to the equipment with the following symbol.
Electromagnetic Fields
Warning: Do not use the System where there is a strong electromagnetic field (e.g. Magnetic Resonance, wireless devices, microwaves emitting devices, etc.).
Mechanical Forces
Caution: While pressing buttons or touching the system, do not use hard or pointed objects or apply excessive force otherwise damage to the equipment will occur.
Connection and Disconnections
Caution: Do not connect/disconnect any cable of the System while any component is powered on, otherwise damage to the equipment will occur.
