MEDTRONIC ANNOUNCES FULL MARKET LAUNCH IN U.S. OF EVOLUT™ FX NEXT-GEN TAVR SYSTEM FOR TREATMENT OF SYMPTOMATIC SEVERE AORTIC STENOSIS
TCT 2022: Medtronic Unveils Latest Transcatheter Aortic Valve Replacement Data in Late-Breaking Clinical Science and Presentations
Medtronic today announced the expanded U.S. market release of its newest-generation, self-expanding transcatheter aortic valve replacement (TAVR) system, the Evolut™ FX TAVR system. Evolut FX adds new features to the existing Evolut platform to enhance ease-of-use and predictable valve deployment for physicians. Along with new data being presented at the upcoming 34th Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, the product launch further demonstrates Medtronic’s commitment to advancing TAVR solutions.
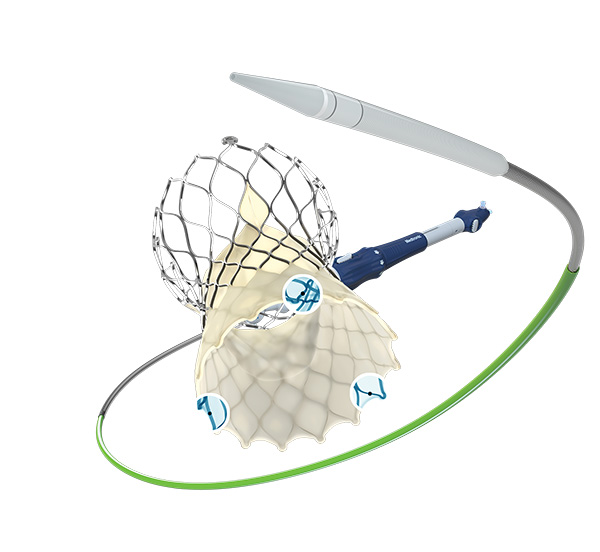
The full commercial release of the Evolut FX system follows U.S. Food and Drug Administration (FDA) approval and a limited roll out earlier this year, which received excellent feedback from participating physicians. With this latest expansion, the Evolut FX system will be available to all commercial TAVR sites across the United States.
The Evolut FX system maintains the industry-leading hemodynamic (blood flow) and durability of the Evolut platform with meaningful design changes. These updates include gold markers built into the frame to provide implanters with direct visualization of commissure alignment; a redesigned catheter tip for a smoother insertion profile; and a more flexible delivery system that features an optimized stability layer for a more stable, predictable deployment. Like its predecessor (Evolut PRO+), the newest system includes four valve sizes for the largest indicated patient treatment range and the lowest delivery profile currently on the market.
“This represents a milestone for our structural heart business, as we look to set new expectations for TAVR delivery systems and optimize outcomes for patients. These innovations will equip physicians with improved implant predictability, ultimately improving the overall reliability of the procedure,” said Jeffrey Popma, M.D., vice president and chief medical officer for the Structural Heart & Aortic business, which is part of the Cardiovascular Portfolio at Medtronic.
TCT 2022: TAVR Data Milestones
Medtronic will announce the findings of four studies, including Late-Breaking Clinical Science on Saturday, September 17, highlighting the latest clinical insights on TAVR technology. The studies build on evidence for the Medtronic TAVR platforms, including new data from the Optimize PRO Study.
Data to be presented at TCT include:
- Late-Breaking Clinical Science: Pathology of Self-Expanding Transcatheter Aortic Valve Failure and Hypo-Attenuated Leaflet Thickening (HALT) on Saturday, September 17th at 2 PM ET with Dr. Yu Sato of CVPath Institute, Inc.
- Adherence to Key Cusp Overlap Steps Reduced Pacemaker Implantation in the Optimize PRO Study on Saturday, September 17th at 8:12 AM ET with Dr. Hemal Gada of UPMC Pinnacle.
- The Evolut Supra-Annular, Self-Expanding Transcatheter Aortic Bioprosthesis Provides Symmetric Scaffolding for Leaflet Coaptation Despite Variable Annular Morphology on Saturday, September 17th at 2:24 PM ET with Dr. Rishi Puri of Cleveland Clinic.
- Transcarotid Versus Transaxillary Access for Transcatheter Aortic Valve Replacement with a Self-Expanding, Supra-Annular Valve on Monday, September 19th at 10:48 AM ET with Dr. Keith B. Allen of St. Luke’s Mid America Heart Institute.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, cranial and spinal robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for all. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
