Medtronic Nexpowder™* Endoscopic Hemostasis System Receives U.S. FDA Clearance
-- Nexpowder™* provides gastroenterologists improved visibility, controls upper gastrointestinal (GI) bleeding effectively and with ease1,2,3 --
-- Nexpowder™* endoscopic hemostasis system showed a lower rebleeding rate and has nearly double the adhesive force of other hemostatic sprays2,3 --
DUBLIN – 9/28/2022 – Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced U.S. Food and Drug Administration clearance of the Nexpowder™* endoscopic hemostasis system. Developed independently by NEXTBIOMEDICAL CO., LTD (Korea) and distributed globally by Medtronic, Nexpowder™* uses a noncontact, nonthermal and nontraumatic hemostatic powder sprayed through a catheter that has a propriety powder-coating technology for minimized clogging which provides improved visibility and control1,2 for treating upper gastrointestinal nonvariceal bleeding. There are more than one million endoscopic hemostasis procedures performed every year in the United States and upper GI bleeding is one of the most common causes, accounting for 75% of all acute GI bleeding cases.4
“We are very excited to bring the innovative, Nexpowder™* system to gastroenterologists. We considered the potential impact on physicians and patients alike, by meeting a clear need to reduce mortality from upper GI bleeding, a condition that causes death for one out of every 1,000 people while also reducing rebleeding, which happens in 20% of all upper GI bleeding cases,” 4 said Gio Di Napoli, president of the Gastrointestinal business, part of the Medical Surgical Portfolio at Medtronic. “The Medtronic GI business is striving – across all products in our portfolio – to create transformative technologies that improve outcomes and change the standard of care.”
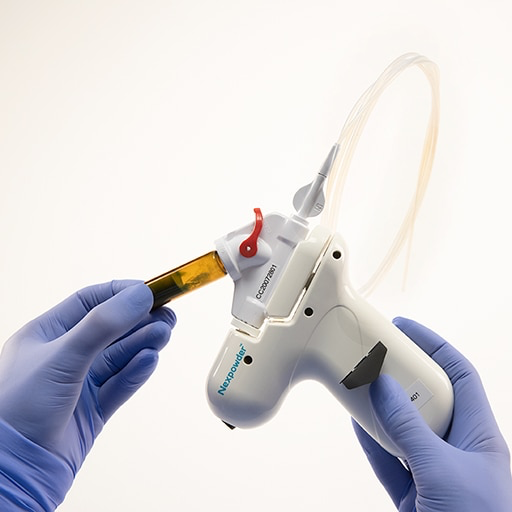
Nexpowder™* is sprayed on to a target site endoscopically through a catheter that connects to a spray handle. Once sprayed, Nexpowder™* immediately forms a muco-adhesive and durable gel upon contact, with or without blood, which degrades in one-to-three days. The spray adheres to the tissue with double the adhesive force of other commercially available products, resulting in a durable hemostatic effect.1,2,3 Nexpowder™* delivers a precise solution for nonvariceal upper GI bleeding with minimal scattering or clogging, enabling direct endoscopic visibility without impairment.
“With a 94% immediate hemostasis rate and a 3.7% rebleeding rate, we’re thrilled to share this effective technology with gastroenterologists,” 2 said Dr. Austin Chiang, M.D., M.P.H., chief medical officer of the Gastrointestinal business at Medtronic. “When we treat patients, we’re looking for immediate and lasting results when it matters most. The Nexpowder™* system is a powerful tool for GI professionals to add to their toolboxes.”
Created with a unique delivery system that doesn’t require CO2 or air compressors, the Nexpowder™* endoscopic hemostasis system combats the inefficiency of clogged catheters and cloudy fields of vision with a proprietary hydrophilic polymer to enable controlled delivery, minimize catheter clogging and maintain endoscopic visibility.3 The system responds to all types of moisture, not only blood.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
About NEXTBIOMEDICAL CO., LTD.
We develop innovative therapeutics and treatment devices. Established in 2014 and headquartered in Incheon, Korea, we are a bio-medical convergence of innovative treatment and medical device development company based on polymer drug delivery technology. Recognized for our technological prowess and commercialization through European CE certification, including GMP certification for facilities and domestic new medical technology, and established sales network through global distributors. In order to increase the efficacy of a single drug or medical device, and to obtain faster approval than a new drug, we are conducting R&D focusing on drug-medical device convergence products that combine two or more approved products into one product. By conducting such R&D more actively, we hope to grow into a company that saves patients' lives and provides new treatment solutions to medical staff. For more information visit www.nextbiomedical.co.kr.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
References:
1 Park JS, Bang BW, Hong SJ, et al. Efficacy of a novel hemostatic adhesive powder in patients with refractory upper gastrointestinal bleeding: a pilot study. Endoscopy. 2019 May;51(5):458-462
2 Park JS, Kim HK, Shin YW, et al. Novel hemostatic adhesive powder for nonvariceal upper gastrointestinal bleeding. Endosc Int Open. 2019 Dec;7(12):E1763-E1767
3 Bang B, Lee E, Maeng J, et al. Efficacy of a novel endoscopically deliverable muco-adhesive hemostatic powder in an acute gastric bleeding porcine model. PLoS One. 2019 Jun 11;14(6):e0216829
4 Antunez, C. Upper Gastrointestinal Bleeding. NCBI Bookshelf, A Service of the National Library of Medicine, National Institutes of Health, July 2020
™* Third party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company.
