Medtronic announces 12-month study results showing DTM™ spinal cord stimulation programming provides long-term, significant pain relief for indicated chronic back pain patients not eligible for spine surgery
NOVA Study: On-label, prospective, multicenter, randomized controlled trial compared DTM™ SCS to conventional spinal cord stimulation
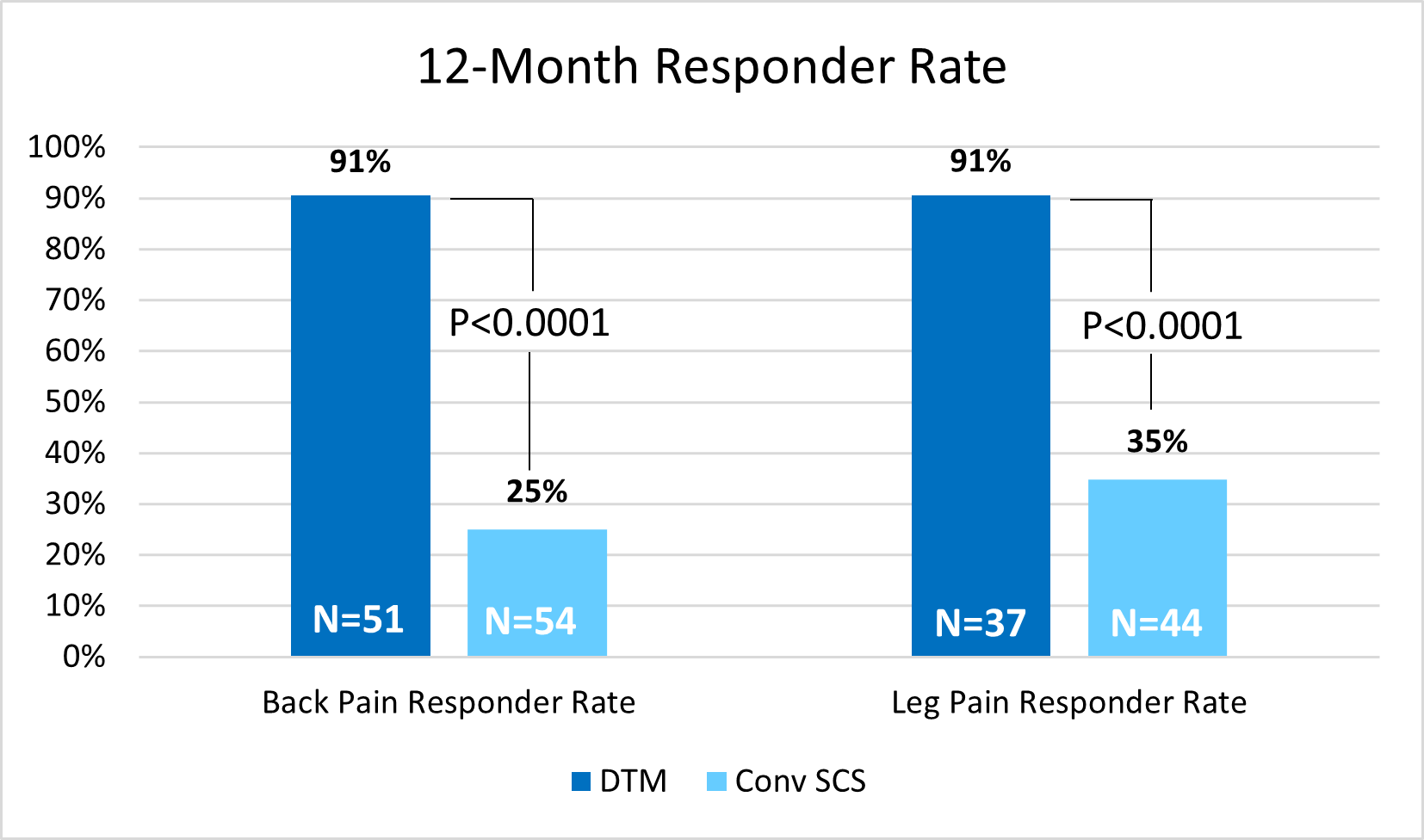
Medtronic, a global leader in healthcare technology, today announced 12-month study results demonstrating that DTM™ SCS programming provides significant, long-term pain relief compared to conventional spinal cord stimulation (SCS) therapy for patients with chronic back pain with degenerative disc disease (DDD), herniated disc (HD), or radicular pain syndrome (RPS) who are not candidates for spine surgery. In the modified intention to treat analysis set (mITT) at 12 months, 91% of patients using DTM™ SCS programming reported at least 50% back pain reduction compared to 25% of patients treated with conventional SCS. Also at 12 months, 91% of patients using DTM™ SCS programming reported at least 50% leg pain reduction compared to 35% of patients treated with conventional SCS. For patients using DTM™ SCS programming, average back pain reduction was 82% at 12 months, an average 6.4 cm reduction of VAS score. Pain scores were measured using the Visual Analog Scale (VAS), a widely used and accepted measure for pain intensity.
“These highly encouraging results show that for indicated patients who are not eligible for spine surgery, attainable, sustainable chronic low back and leg pain relief is possible with DTM™ SCS therapy,” said Dr. Ricardo Vallejo, SGX Medical, Bloomington, IL, and an author of the study. “Not only do these results show significant pain relief, but also reduced disability and a high degree of overall patient satisfaction. For those of us in the field who are treating these patients with limited options, that is music to the ears.”
Additional study findings for per protocol (PP) subjects include:
- Clinically significant reduction in disability, with 95% of subjects receiving DTM™ SCS programming reporting moderate to minimal disability at 12 months compared to 52% at baseline.
- Measuring patient global impression of change (PGIC), 89% of patients felt very much improved or much improved with DTM™ SCS programming at their 12-month visit.
- Measuring patient satisfaction, 93% of patients reported that they were either very satisfied or satisfied with their DTM™ SCS programming at 12 months.
- The study met its primary endpoint at 3 months, defined as the percentage of individual responders (patients reporting ≥50% back pain relief vs. baseline) to the randomly assigned treatment (DTM™ SCS vs. conventional SCS). Individual responder rate for the DTM SCS programming arm was 94% at 3 months, compared to a 36% responder rate for conventional SCS.
“These strong results go hand in hand with our previous RCT, which showed 84% of patients with chronic back and leg pain treated with DTM™ SCS programming on the Intellis™ platform reported at least a 50% reduction in pain at 12 months1,” said David Carr, vice president and general manager, Pain Interventions, which is part of the Neuroscience Portfolio at Medtronic. “Medtronic remains committed to continuously advancing clinical evidence and providing relief for more people living with chronic pain, including those who are unable to consider surgery.”
DTM™ SCS is a proprietary Medtronic waveform based on years of preclinical research. The waveform may engage a novel mechanism that modulates both neurons and glial cells, expanding the understanding of SCS mechanisms of action. Glial cells outnumber neurons in the spinal cord by 12:1 and their role in pain have been explored in research for more than 20 years.2,3,4
The study was conducted across 20 centers in the United States. Patients at 6 months were able to cross over from the conventional SCS arm to the DTM SCS arm if they weren’t experiencing desired results, with 48% choosing to do so. All patients were implanted with the Intellis™ spinal cord stimulator. Study results were first presented at ASRA Pain Medicine 22nd Annual Pain Medicine Meeting on November 10-11 in a poster titled “DTM™ SCS for Indicated Chronic Back Pain Patients Non-Eligible for Spine Surgery: US RCT Outcomes,” and the study was sponsored by SGX NOVA. The study was presented by lead investigator Dr. Thomas White, Procura Pain and Spine, Houston, TX.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com, and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1 Fishman M, Cordner H, Justiz R, et al. 12-Month Results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021; 00: 1– 12. doi: 10.1111/papr.13066
2 Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009 Jan;10(1):23-36.
3 Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010 May-Jun;10(3):167-84.
4 De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: Path to CNS centralization and chronic pain. Pain. 2006; 122:17-21.
