Medtronic is the first and only company to receive FDA indication for bifurcation percutaneous coronary intervention with a drug-eluting stent
Bifurcation using Onyx Frontier DES Medtronic announced today that it has received Food and Drug Administration (FDA) approval for the treatment of non-left main bifurcation lesions utilizing the...
Medtronic announced today that it has received Food and Drug Administration (FDA) approval for the treatment of non-left main bifurcation lesions utilizing the provisional bifurcation stenting technique (using a single stent to treat the bifurcation) with the Onyx Frontier™ drug-eluting stent (DES) and the Resolute Onyx™ DES.1 This indication will allow Medtronic to provide a robust portfolio of medical education and procedural training for physicians performing percutaneous coronary interventions (PCIs) in patients with bifurcation lesions.
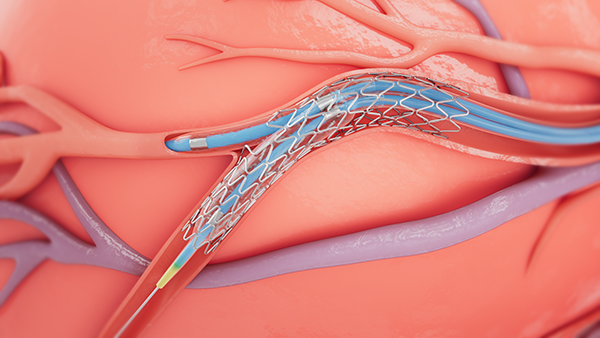
Bifurcation lesions occur when plaque builds up around the junction of two coronary arteries - where one branches off another. These lesions are considered challenging to treat because of anatomical variations in the vessels and the difficulty associated with reaching the side branches. The treatment of bifurcation lesions is commonly associated with lower success rates and increased rates of long-term adverse cardiac events,2 and since patients with bifurcation lesions often present unique challenges for interventional cardiologists, stent attributes – like those found in the Onyx Frontier and Resolute Onyx drug-eluting products - become important to optimize the treatment of these lesions.
“The bifurcation expanded indication is yet another exciting milestone for our Coronary business this year,” said Jason Weidman, senior vice president and president of the Coronary & Renal Denervation business, which is part of the Cardiovascular Portfolio at Medtronic. “As the first and only medical device company to offer this indication to U.S. interventional cardiologists, Medtronic remains committed to investing in DES technology, clinical evidence, and physician education. We are looking forward to helping even more physicians access the tools they need to give their patients best-in-class care.”
Medtronic is the only company to offer drug-eluting stents featuring a single-wire design – a manufacturing process in which a single strand of wire is formed into a sinusoidal wave to construct a stent. This design allows for the optimized treatment of complex bifurcation anatomy by providing excellent vessel conformability, easier side branch access, and the option to open the stent cell while maintaining consistent stent scaffolding.2,3 In addition to an ideal stent design, Onyx Frontier and Resolute Onyx DESs are backed by clinical evidence from the Resolute Onyx Bifurcation Study. Resolute Onyx DES demonstrated low event rates, beating the performance goal for the primary endpoint of target vessel failure at one year.4 The positive results from this study support the safety and efficacy of Resolute Onyx DES and Onyx Frontier DES in non-left main bifurcation lesions using the provisional stenting technique.
Both the Onyx Frontier and Resolute Onyx DESs are FDA and CE Mark approved.
Contact:
Lauren Mueller Doran
Public Relations
+1-763-285-9053
1Onyx Frontier DES IFU
2Sawaya, F., et. al. Contemporary approach to coronary bifurcation lesion treatment. Journal of the American College of Cardiology. September 2016; 18: 1861-1878. http://interventions.onlinejacc.org/content/9/18/1861(opens new window)
3Data on file at Medtronic. May not be indicative of clinical performance.
4Price M. One year Clinical Outcomes in Patients with coronary bifurcation lesions: results from the Resolute Onyx Bifurcation Study. Presented at ACC 2021.
