Medtronic receives CE Mark Approval for innovative technologies in pulsed field and cryoablation: PulseSelect™ Pulsed Field Ablation System and Nitron CryoConsole™ to advance treatment of atrial fibrillation
Simplicity, efficiency, efficacy with unmatched safety data to set a new standard in catheter ablation
Medtronic has received CE (Conformité Européenne) Mark for the PulseSelect Pulsed Field Ablation (PFA) System and the Nitron CryoConsole.
The PulseSelect System is designed to treat atrial fibrillation (AFib) effectively, efficiently, and safely, with a new ablation modality that uses pulsed electric fields to isolate the pulmonary veins. This addition to the Medtronic PFA portfolio follows the March European approval of the Affera™ Mapping and Ablation System and positions Medtronic as the only company with both single shot and focal PFA options to meet different patient and clinician needs. Additionally, the Nitron console builds upon the legacy of Medtronic’s Cryo franchise, elevating and optimizing the workflow for cryoballoon ablation.
“With multiple CE Mark milestones, today’s announcement demonstrates our commitment to innovation and building a strong electrophysiology portfolio,” said Rebecca Seidel, president of the Cardiac Ablation Solutions business, which is part of the Cardiovascular Portfolio. “These milestones are part of our investment in the future of our cryoablation franchise with Nitron, as well as the future of pulsed field ablation with PulseSelect, following our more than ten years of scientific research and development.”
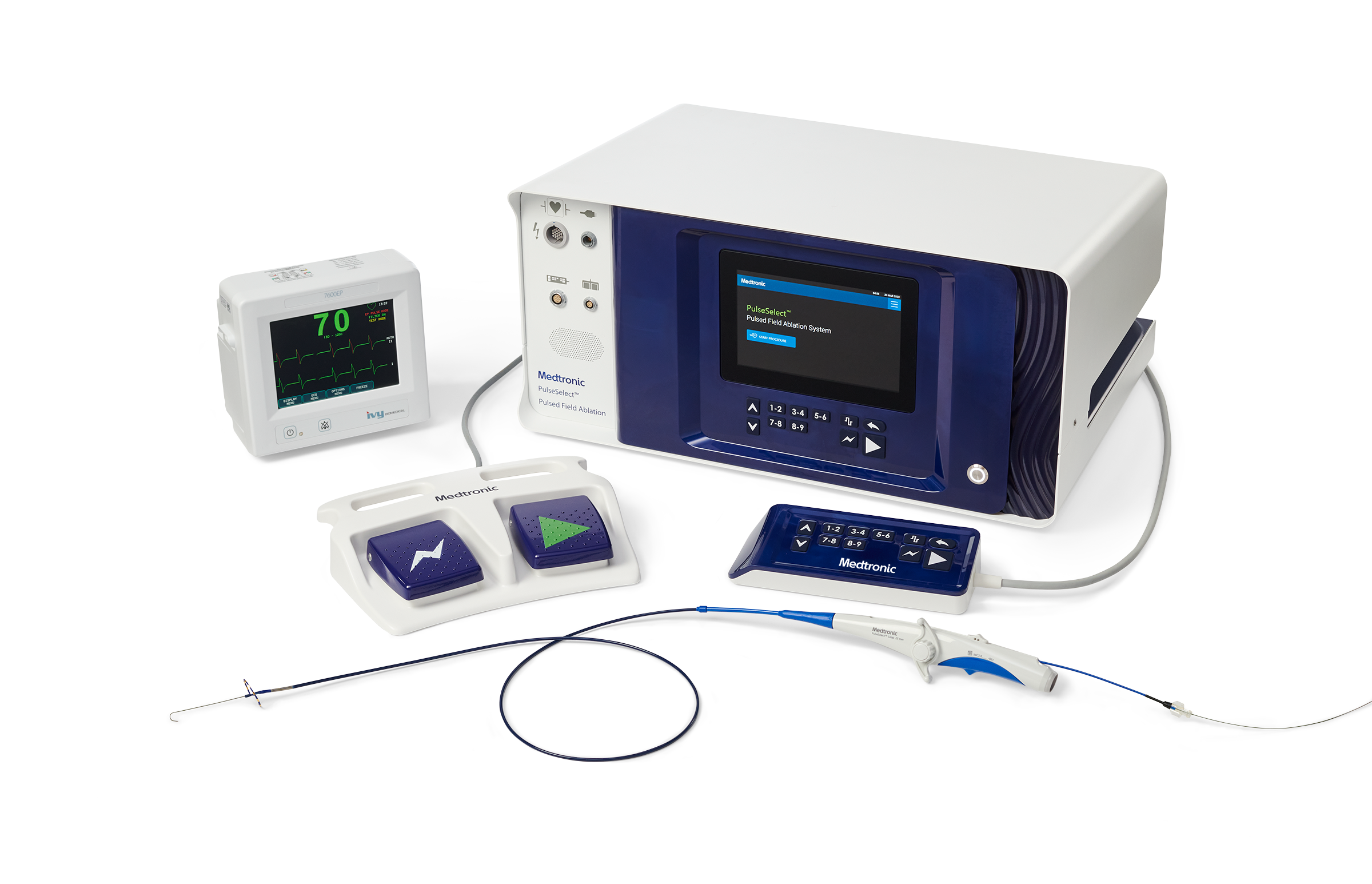
About PulseSelect
PulseSelect uses a non-thermal approach to preferentially target the pulmonary veins for ablation, avoiding unwanted injury to surrounding structures, which is a risk of current ablation technologies (thermal energy sources). The catheter utilizes a proprietary biphasic waveform, a unique built-in over-the-wire design and 20-degree forward tilt to support procedural maneuverability, reliability, and safety, as demonstrated by one of the safest global ablation clinical trials to-date, PULSED AF. Based on data from this trial, the catheter has demonstrated efficient pulmonary vein isolation with an average of 30 seconds of total energy delivery time to isolate all veins.
“The electrophysiology community is eagerly awaiting new innovation to enhance the safety and efficiency of AFib ablation. PulseSelect is specifically engineered to deliver pulsed field energy safely and maneuvers well, with great overall control of the energy applications,” said Lucas V.A. Boersma, M.D., Ph.D., cardiologist at St. Antonius Hospital, Department of Cardiology, Nieuwegein, The Netherlands, and Professor of Cardiology, Amsterdam University Medical Centers, University of Amsterdam, The Netherlands. “The safety profile shown by the PulseSelect System in its global pivotal study is one of the best compared to today’s standard of care for catheter ablation technologies.”
The PulseSelect system also includes the following:
- Designed as a plug-and-play system, PulseSelect can be used with any mapping system or with just fluoroscopy.
- Built-in safety features such as a phrenic nerve test pulse, act as a non-therapeutic low voltage pulse that provides a preemptive assessment of catheter proximity to the phrenic nerve prior to delivering a therapeutic application.
- Fixed spacing for the nine-electrode catheter produces a predictable and consistent electric field for contiguous ablation. In addition to ablation, the nine electrodes can also be used for pacing and sensing.
- The small, 9Fr bidirectional catheter enhances maneuverability and access to various anatomical structures and is compatible with a 10Fr sheath.

About Nitron
The next-generation Nitron console supports the commercially available Arctic Front™ and Freezor™ family of Cardiac Cryoablation Catheters and incorporates a number of new features, including:
- Automated and customizable key procedural data captured real-time and post-procedure in an exportable case summary report.
- Optimized workflow that features a touchscreen interface, wired remote control, and mirrored Nitron CryoConsole display on external lab monitors.
- Enhanced graphical user interface (GUI) with intuitive CryoConsole operation guidance, custom user profiles, and visual indicators of procedure parameters like TTI and previous freeze trace.
“In addition to the state-of-the-art Sphere-9 Catheter with the Affera Mapping and Ablation System, we are thrilled to see the continuous innovation of our legacy Cryoablation with Nitron alongside the approval of single-shot PulseSelect PFA system,” said Khaldoun Tarakji, MD MPH, chief medical officer of the Cardiac Ablation Solutions business at Medtronic. “Every patient deserves the best care. What motivates all of us at Medtronic is the privilege of serving patients by empowering electrophysiologists globally with the safest and most effective ablation technologies that seamlessly integrate with their workflows and enable them to tailor therapy based on their patients’ needs.”
Both PulseSelect and Nitron will be commercially available in select European countries early next calendar year.
About PULSED AF
CE Mark approval follows the PULSED AF global study, which met its safety and effectiveness endpoint. Data was presented as late breaking science at the American College of Cardiology's Annual Scientific Session Together with World Congress of Cardiology (ACC.23/WCC) and was simultaneously published in Circulation. PULSED AF uniquely studied two patient populations (paroxysmal and persistent). PulseSelect exceeded its safety performance goal with an adverse event rate of 0.7%, one of the lowest adverse event rates of any global AFib ablation clinical trial to date. There were no esophageal events, instances of pulmonary vein stenosis, or phrenic nerve injury. PULSED AF exceeded the threshold for its efficacy performance goal. Further, clinical success, freedom from recurrence of any symptomatic atrial arrhythmias, was at least 80% for both paroxysmal and persistent patient cohorts. For more information on PULSED AF, visit Medtronic.com/PFA.
Contacts:
Allison Kyriagis
Public Relations
Medtronic
+1-612-750-6061
Ryan Weispfenning
Investor Relations
Medtronic
+1-763-505-4626
