Medtronic receives CE Mark for Evolut™ FX TAVI System for treatment of severe aortic stenosis
Next-Gen Evolut FX TAVI system enhances procedure ease-of-use, visualization, and predictability
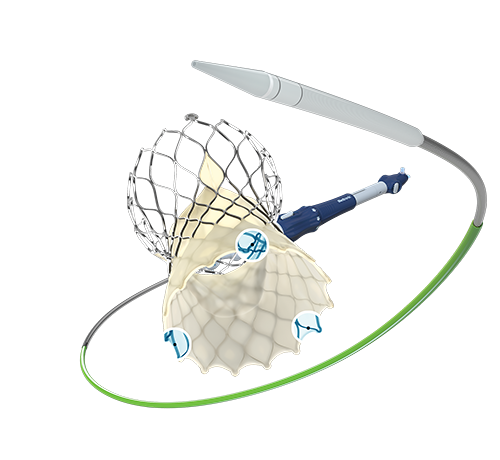 Medtronic plc (NYSE:MDT) today announced it has received CE (Conformité Européenne) Mark for the Evolut™ FX transcatheter aortic valve implantation (TAVI) system, the newest-generation TAVI system of the CoreValveTM/Evolut TAVI platform. The Evolut FX system is designed to enhance ease-of-use and provide greater precision and control throughout the procedure for clinicians treating patients with severe aortic stenosis. The CoreValve/Evolut platform is the only TAVI platform to demonstrate a durability advantage over SAVR at fiveand 10 years in a randomized trial.1-2
Medtronic plc (NYSE:MDT) today announced it has received CE (Conformité Européenne) Mark for the Evolut™ FX transcatheter aortic valve implantation (TAVI) system, the newest-generation TAVI system of the CoreValveTM/Evolut TAVI platform. The Evolut FX system is designed to enhance ease-of-use and provide greater precision and control throughout the procedure for clinicians treating patients with severe aortic stenosis. The CoreValve/Evolut platform is the only TAVI platform to demonstrate a durability advantage over SAVR at fiveand 10 years in a randomized trial.1-2
The Evolut FX system incorporates the same supra-annular, self-expanding valve design as the CoreValve/Evolut platform which has shown to have a superior hemodynamic performance compared to surgical aortic valve replacement (SAVR) at one-year across three separate, large-scale, randomized controlled trials.3, 4, 5 The fourth-generation Evolut technology is equipped with gold markers built into the frame to provide implanters with direct visualization of depth and valve leaflet orientation during implant. In addition, the Evolut FX system incorporates a redesigned catheter for a smoother insertion profile and a more flexible delivery system for a stable, predictable deployment.
“With the latest Evolut FX system, we are elevating the precision, control and predictability of transcatheter aortic valve replacement procedures for patients with severe aortic stenosis,” said Danny Dvir, M.D., interventional cardiologist and director of Interventional Cardiology and Cath Labs at Shaare Zedek Hospital Canter in Jerusalem, Israel. “The system provides physicians with an innovative solution to meet the needs of a patient population desiring to get back to their active lifestyles sooner.”
Severe aortic stenosis occurs when the aortic valve leaflets become stiff and thickened and have difficulty opening and closing, making the heart work harder to pump blood to the rest of the body. Severe aortic stenosis often reduces a patient's quality of life and limits their daily activities. If left untreated, patients with symptomatic severe aortic stenosis can die from heart failure in as little as two years.6
“This exciting milestone helps us continually enhance a trusted platform to better respond to clinicians' needs making TAVI procedures easier to visualize and more predictable for heart teams,” said Jeffrey Popma, M.D., vice president and chief medical officer for the Coronary & Renal Denervation business and the Structural Heart & Aortic business, which are part of the Cardiovascular Portfolio at Medtronic. “Receiving CE Mark for the Evolut FX system highlights our commitment to providing minimally invasive treatment options globally for patients experiencing severe aortic stenosis.”
The Evolut FX system is indicated for severe aortic stenosis in adult patients across all risk categories (extreme, high, intermediate, and low) in the European Union and is indicated for symptomatic severe aortic stenosis patients across all risk categories in the U.S. The Evolut FX system is expected to be commercially available across Europe in the coming weeks as teams and physicians are trained on this new technology.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Contacts:
Rachel Murray
Public Relations
+1-651-332-3286
Ryan Weispfenning
Investor Relations
+1-763-505-4626
1 O’Hair D, Yakubov SJ, Grubb KJ, et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiology. 2023;8(2):111. doi:10.1001/jamacardio.2022.4627
2 Jørgensen TH, Ten-year follow-up after transcatheter or surgical aortic value implantation in severe aortic valve stenosis. European Society of Cardiology Congress 2023. 2023. https://esc365.escardio.org/presentation/272247?resource=video
3 Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. New England Journal of Medicine. 2014;370(19):1790-1798. doi:10.1056/nejmoa1400590
4 Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. New England Journal of Medicine. 2017;376(14):1321-1331. doi:10.1056/nejmoa1700456
5 Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. New England Journal of Medicine. 2019;380(18):1706-1715. doi:10.1056/nejmoa1816885
6 Ross J Jr, Braunwald E. Aortic stenosis. Circulation. July 1968; 38(1 Suppl):61-67.
