Delivering hope for patients with chronic kidney disease
Medtronic is celebrating National Kidney Month by recognizing patients' stories of resiliency and hope
Dez Delatorre was devastated to learn he had advanced kidney disease in 2007.

He’d witnessed several family members battle the disease and the thought of being placed on dialysis sounded “like a death sentence” to the former police officer.
“I had quite a few relatives who were not proactive managing their kidney disease,” Delatorre said. “There were quite a few, including my own mother, who had what I call ‘the dark side of kidney disease’ because by the time they realized that their kidneys failed them, it was too late.”
Delatorre’s battle with kidney disease, however, was far from over. He started dialysis a few years ago and it’s been a life-changing experience, but not in the way he had feared because of an innovative Medtronic technology.
Today, Delatorre is one of an estimated 37 million adults in the United States living with chronic kidney disease.1 When a person’s kidneys fail, only dialysis and transplant can save them.
But as Delatorre’s story illustrates, chronic kidney disease patients are not without hope. In March, which is National Kidney Month, Medtronic is celebrating these patients and some of the innovative technologies to treat their disease.
Living well on dialysis
Dialysis is a fact of life for many chronic kidney disease patients. While it can provide life-saving treatment, dialysis can also be challenging as it requires patients to make several lifestyle changes.
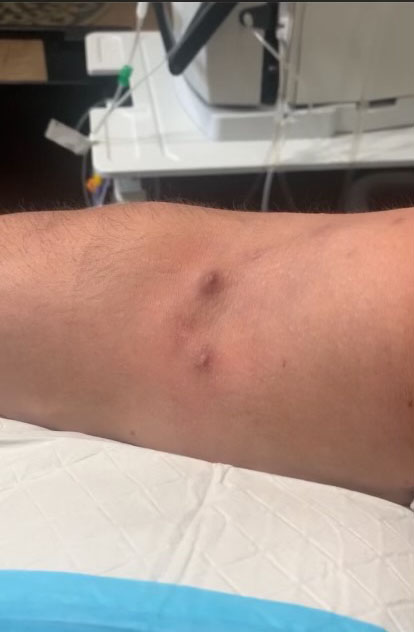
To prepare patients for dialysis, a clinician must create a vascular access point where blood will ultimately be removed, circulated through the dialysis machine, and then returned to the body. An arteriovenous (AV) fistula is a common type of access point. To create one, a surgeon connects an artery to a vein, most often in the arm.
Patients who receive AV fistulas often feel the need to cover their arms with long sleeves because the site of the suture can have scarring and/or bulging veins following the procedure. But our Ellipsys™ Vascular Access System is giving patients like Delatorre a new way to think about their AV fistulas.
The system is a unique, single catheter non-surgical option for clinicians creating AV fistulas. This minimally invasive procedure replaces surgery and has evidence out to two years demonstrating its ability to reduce lengthy recovery times and maintain blood flow.2
“Until recently, the traditional alternative to create AV fistulas was an invasive surgery, which is associated with high failure rate and long recovery and maturation times,” said Dave Moeller, President of the Peripheral Vascular Health operating unit at Medtronic. “The Ellipsys system addresses a critical treatment problem that exists today – getting AV fistulas created faster for patients in desperate need of this lifeline.”
Since Ellipsys is minimally invasive, patients leave with just a small bandage and no long-term scarring.3
Delatorre, who hopes to receive a kidney donation someday, says he can comfortably wear a T-shirt without concern of his appearance.
“You can look at my arm and see little bumps, but that’s it,” he said. “I look normal. It doesn’t look like something goes in there.”
An ‘angel’ donates a kidney
The Ellipsys system is at the center of another remarkable patient story. This one belongs to Jeff Cooper, a retired emergency medical technician diagnosed with polycystic kidney disease.
Like Delatorre, Cooper resisted the idea of dialysis. His father was on dialysis for 14 years and he recalled the toll it took on him which included bulging veins in his arm. So, when Cooper heard about the Ellipsys procedure, he was intrigued.
Cooper said he was amazed that the procedure was so easy, and after his recovery, his arms were not left lumpy or bumpy. He faithfully underwent dialysis treatment for more than a year while waiting for a kidney transplant.
Then late last year, he got the call that changed his life forever. A stranger had agreed to donate a kidney.
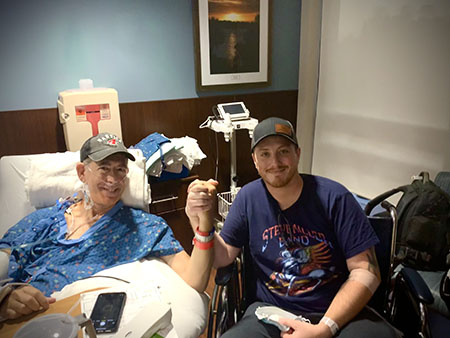
The stranger turned out to be Guy Kitchens, a police officer who lives in a nearby city. Kitchens had learned about Cooper on social media and when his 8-year-old daughter, Addie, encouraged him to donate his kidney, he couldn’t refuse.
Today, Cooper says he feels like a kid again. His kidney function is improving, and doctors are dialing back his medication. Even better? His burgeoning relationship with Kitchens with whom he talks to almost daily.
“I’ve got a little brother now and another extended family,” Cooper said. “I’ve got a living angel in my life.”
Small device making a big impact for pediatric dialysis patients
Chronic kidney disease has many causes, such as blood loss, medicines, urinary blockage, genetic disease, or infections. And unfortunately, it can strike at any age.
Until recently, critically ill infants requiring continuous renal replacement therapy (CRRT) had few treatment choices beyond devices designed and approved for adults. But a new Medtronic technology is now providing treatment for the tiniest patients.
Launched in late 2020, the Carpediem™ system is the first and only pediatric and neonatal dialysis machine approved in the United States. It is designed to provide CRRT to patients weighing between 5.5 and 22 pounds.
The first Carpediem system was used at the Cincinnati Children’s Hospital Medical Center where Dr. Stuart L. Goldstein has led efforts to raise awareness about the critical need for safe pediatric-specific dialysis.
“Small device. Big impact,” said Goldstein, M.D., professor of pediatrics and director of the hospital’s Center for Acute Care Nephrology. “Our unparalleled collaborative journey with Medtronic has facilitated movement of the provision of care needle towards attainment of the highest possible infant kidney health outcomes."
Citations
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2021. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2021.
- Beathard GA, Litchfield T, Jennings WC. Two-year cumulative patency of endovascular arteriovenous fistula. J Vasc Access. May 2020;21(3):350-356
- Hull JE, Jennings WC, Cooper RI, Waheed U, Schaefer ME, Narayan R. The Pivotal Multicenter Trial of Ultrasound-Guided Percutaneous Arteriovenous Fistula Creation For Hemodialysis Access. JVIR 2018 Feb.
Important Safety Information
Indications
The Ellipsys™ system is indicated for the creation of a proximal radial artery to perforating vein anastomosis via a retrograde venous access approach in patients with a minimum vessel diameter of 2.0 mm and less than 1.5 mm of separation between the artery and vein at the fistula creation site who have chronic kidney disease requiring dialysis.
Contraindications
The Ellipsys™ system is contraindicated for use in patients with target vessels that are <2 mm in diameter. The Ellipsys™ system is contraindicated for use in patients who have a distance between the target artery and vein > 1.5 mm
Warnings
- The Ellipsys™ system has only been studied for the creation of an AV fistula using the proximal radial artery and the adjacent perforating vein. It has not been studied in subjects who are candidates for surgical fistula creation at other locations, including sites distal to this location.
- The Ellipsys™ system is not intended to treat patients with significant vascular disease or calcification in the target vessels.
- The Ellipsys™ system has only been studied in subjects who had a patent palmar arch and no evidence of ulnar artery insufficiency.
- Use only with the Ellipsys™ system power controller, Model No. AMI-1001.
- The Ellipsys™ catheter has been designed to be used with the 6 F Terumo Glidesheath Slender™*. If using a different sheath, verify the catheter can be advanced through the sheath without resistance prior to use.
- Use ultrasound imaging to ensure proper placement of the catheter tip in the artery before retracting the sheath, since once the distal tip of the catheter has been advanced into the artery, it cannot be easily removed without creation of the anastomosis. If the distal tip is advanced into the artery at an improper location, complete the procedure and remove the catheter as indicated in the directions for use. It is recommended that a follow-up evaluation of the patient is performed using appropriate clinical standards of care for surgical fistulae to determine if any clinically significant flow develops that require further clinical action.
Precautions
- This product is sterilized by ethylene oxide gas.
- Additional procedures are expected to be required to increase and direct blood flow into the AVF target outflow vein and to maintain patency of the AVF. Care should be taken to proactively plan for any fistula maturation procedures when using the device.
- In the Ellipsys™ system study, 99% of subjects required balloon dilatation (PTA) to increase flow to the optimal access vessel and 62% of subjects required embolization coil placement in competing veins to direct blood flow to the optimal access vessel. Prior to the procedure, care should be taken to assess the optimal access vessel for maturation, the additional procedures that may be required to successfully achieve maturation, and appropriate patient follow-up. Please refer to the “Arteriovenous Fistula (AVF) Maturation” section of the labeling for guidance about fistula flow, embolization coil placement, and other procedures to assist fistula maturation and maintenance.
- The Ellipsys™ system is intended to only be used by physicians trained in ultrasound guided percutaneous endovascular interventional techniques using appropriate clinical standards for care for fistula maintenance and maturation including balloon dilatation and coil embolization.
- Precautions to prevent or reduce acute or longer-term clotting potential should be considered. Physician experience and discretion will determine the appropriate anticoagulant/antiplatelet therapy for each patient using appropriate clinical standards of care.
Potential Adverse Events
Potential complications that may be associated with creation and maintenance of an arteriovenous fistula include, but may not be limited to, the following:
- Total occlusion, partial occlusion or stenosis of the anastomosis or adjacent outflow vein
- Stenosis of the central AVF outflow requiring treatment per the treatment center’s standard of care
- Failure to achieve fistula maturation
- Incomplete vessel ligation when using embolization coil to direct flow
- Steal Syndrome
- Hematoma
- Infection or other complications
- Need for vessel superficialization or other maturation assistance procedures
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Important Information: Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device.
***********************************************************************************************
The CARPEDIEM System is indicated for use in acute kidney injury or fluid overloaded patients requiring hemodialysis or hemofiltration therapy. It is intended to provide continuous renal replacement therapy (CRRT) to patients weighing between 2.5 and 10 kilograms (5.5 and 22 pounds)
Limitations for Use: Do not use CARPEDIEM if patient treatment requires performance outside its operating and accuracy range as well as the operating limits specified in the CARPEDIEM System Operator’s Manual. The CARPEDIEM system allows anticoagulation method with heparin only.
Contraindications: Patients with a history of allergic reactions to polyethersulfone should not be treated using the Carpediem system. The choice to perform CRRT must consider the balance between risk and benefit for patients that have general contraindications to using an
extracorporeal therapy. These factors include: hemodynamic instability, contraindication to suitable anticoagulation, low platelet count, lack of suitable placement for vascular access.
Warnings: Replacement fluid for hemofiltration should be prescribed by a physician and should be commercially available or prepared in the hospital pharmacy, labeled sterile and for intravenous injection. The device is intended to be used by trained clinicians who are experienced in administrating and managing continuous renal replacement therapy (CRRT) in in critically ill pediatric patients.
Cautions: Read all Instructions for Use carefully prior to use.
For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with the system and its components, refer to the Carpediem System Operator’s Manual and the respective system component’s Instructions for Use. Rx Only.
L001-03212022

