Optimized procedure and care pathway with Medtronic Evolut™ TAVR System demonstrates excellent valve performance and procedural outcomes
Mid-term results of Optimize PRO clinical study reinforces benefits of predictability and control using cusp overlap technique
Medtronic today announced results of 400 patients treated in the United States and Canada from the main cohort of the Optimize PRO clinical study evaluating valve performance and procedural outcomes associated with an “optimized” pre- and post-procedural TAVR care pathway, utilizing the cusp overlap technique to deploy the Evolut valves. The cusp overlap technique is designed to help implanters assess and achieve the target implant depth to reduce interaction with the conduction system. Findings were presented as a featured Hotline Trial at EuroPCR 2022.
The study results show that the cusp overlap technique led to more predictability and control resulting in a single-digit pacemaker implantation rate (9.2%1), a low 30-day mortality rate (0.8%), median length of hospital stay of one day, and upon discharge, no cases of moderate/severe paravalvular leak (PVL) (0%) and 78% of patients had none/trace PVL. The lowest pacemaker implantation rates were observed when all steps of the cusp overlap technique were followed.
"The results demonstrate significant improvement for TAVR with the Evolut valve, some of the best results in an Evolut trial we have ever seen," said Kendra Grubb, M.D., surgical director of the Structural Heart and Valve Center at Emory University in Atlanta, and co-principal investigator in the Optimize PRO study who presented the data at the meeting. “Adopting the cusp overlap technique and clinical pathways resulted in single-digit pacemaker rates out to 30 days, next-day discharge in the majority of the patients, and remarkably low rates of paravalvular leak.”
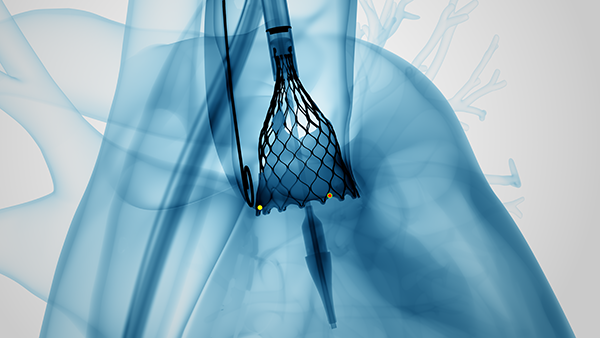
The Optimize PRO study utilizes a TAVR care pathway to evaluate common practices and shared experiences such as conscious sedation and early mobilization. A conduction disturbance pathway evaluates efficiencies and heart team considerations for monitoring and managing patients with conduction disturbance who might be considered for a pacemaker post TAVR.
“We are committed to improving the TAVR care pathway with simple and repeatable techniques that implanting centers can use to help improve procedure efficiencies and success,” said Neil Yanke, vice president and general manager of the Structural Heart business, which is part of the Cardiovascular Portfolio at Medtronic. “The findings presented today – coupled with our recent data presented at ACC 2022 establishing the CoreValve/Evolut TAVR systems as the first and only TAVR platform to demonstrate significantly lower rates of structural valve deterioration (SVD) vs. SAVR at five years – help to further establish the robust clinical evidence physicians need to make the best decisions for their patients.”
The post-market, prospective, multi-center study is evaluating outcomes associated with procedure-related techniques and post-procedure transcatheter aortic valve replacement (TAVR) when using the self-expanding, supra-annular Evolut™ PRO and PRO+ TAVR systems in patients with symptomatic severe aortic stenosis. The current interim analysis includes 400 main cohort patients and 104 roll-in patients at up to 46 sites in the United States and Canada. The primary endpoint includes the rate of all-cause mortality or all-stroke at 30 days with secondary endpoints of discharge time, percent of patients with more than moderate aortic regurgitation, and the rate of pacemaker implant for new-onset or worsening conduction disturbance at 30 days. The study will also evaluate rehospitalization rates and discharge time at 30 days and one year.
The Medtronic CoreValve™ Evolut™ R, CoreValve™ Evolut™ PRO, and Evolut™ PRO+ systems are indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be appropriate for the transcatheter heart valve replacement therapy.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Contacts:
Wendy Dougherty
Public Relations
+1-763-381-1204
Ryan Weispfenning
Investor Relations
+1-763-505-4626
1 Includes main cohort and roll-in patients.
