Updated ClosureFast™ radiofrequency ablation catheter receives U.S. FDA 510(k) clearance for treatment of chronic venous insufficiency
The ClosureFast™ catheter update brings new features including lower 6F profile and longer heating element for greater efficiency
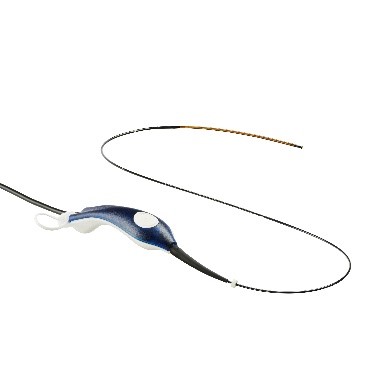
Medtronic today announced that an updated ClosureFast™ radiofrequency ablation (RFA) catheter in a lower 6F profile is now available in the U.S. following 510(k) clearance from the U.S. Food and Drug Administration (FDA).
The ClosureFast procedure is intended to treat chronic venous insufficiency (CVI), a progressive medical condition that affects the veins in the legs that help return blood to the heart. It is estimated that approximately 30 million people suffer from chronic venous insufficiency in the U.S. alone”.1,2
“For patients with mild tortuosity in their saphenous veins, where I would normally use a wire or two access points, I’ve found that the new ClosureFast 6F system glides through more easily than the 7F system, providing a better periprocedural experience for the patient,” said Dr. Misaki Kiguchi, vascular surgeon at MedStar Heart and Vascular Institute.
The ClosureFast procedure uses radiofrequency ablation – or heat – to close the diseased vein, which redirects blood flow to healthy veins, relieving symptoms. With 2.5 million+ patients treated,3 the ClosureFast procedure is the global market leader in RFA for the treatment of chronic venous insufficiency and has 200+ published clinical studies and articles.4
ClosureFast 6F builds upon the technology’s proven platform. New features in the updated ClosureFast RFA catheter include:
- A lower profile 6F catheter designed for better flexibility, easier navigation, and greater kink resistance, even in tortuous veins5
- Increased length of the heating element (from 7 cm to 8 cm) for greater procedural efficiency
“Offering the ClosureFast catheter in a lower 6F profile is part of our commitment to provide meaningful product advancements for patients,” said David Moeller, SVP and President of Peripheral Vascular Health at Medtronic. “We listened to our customers’ feedback to evolve this trusted, market leading technology. The new features are designed to provide advantages in technical performance and procedural efficiency.”
In collaboration with leading clinicians, researchers, and scientists worldwide, Medtronic offers a broad range of innovative medical technology for the interventional and surgical treatment of cardiovascular and peripheral vascular diseases. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world.
For more information, contact:
Contacts:
Laura Hennen
Public Relations
+1-763-213-3034
Ryan Weispfenning
Investor Relations
+1-763-505-4626
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.medtronic.com.
References
- Gloviczki P, et al. J Vasc Surg. 2011;53:2S-48S.
- Eberhardt R, et al. Circulation. 2005; 111:2398-2409.
- ClosureFast Procedures. Medtronic data on file, 2022.
- Medtronic ClosureFast Procedure Publications. Medtronic data on file, 2022.
- Compared to ClosureFast 7F catheter and Venclose™* catheter in bench testing. Bench test data on file at Medtronic. Bench tests may not be indicative of clinical performance. N=15 Venclose catheters and 30 each of ClosureFast 7F and ClosureFast 6F catheters used.
