Month Year
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
Month Year
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Mar 5, 2023
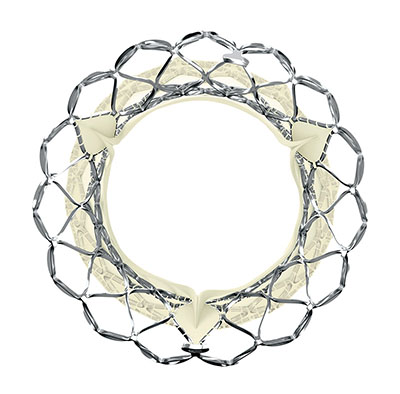
ACC.23/WCC Late-Breaking Clinical Trial: Evolut™ is only TAVR to show excellent outcomes in low-risk patients at 3 years
-
Feb 27, 2023
CRT 2023 Late-Breaking Data: CoreValve/Evolut™ platform demonstrates significantly lower bioprosthetic valve dysfunction compared to surgery at five years
-
Feb 22, 2023
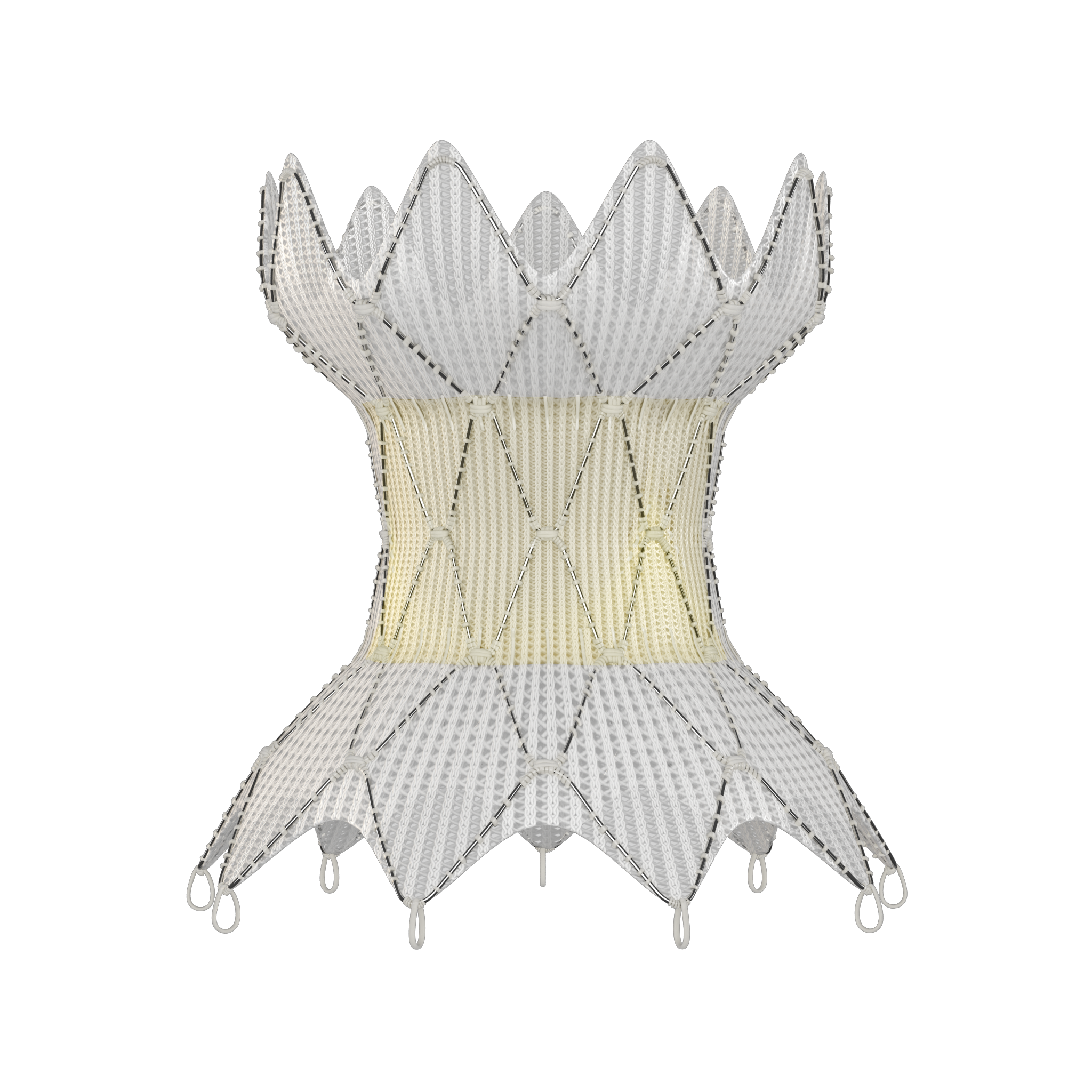
Harmony™ Transcatheter Pulmonary Valve System is the first FDA-approved minimally invasive valve designed for the right side of the heart
-
Jan 30, 2023
Medtronic plc (NYSE: MDT) recently announced the appointment of Michael Blackwell as Vice President of India Medtronic Pvt. Ltd., effective January 2023. In this role, he will be responsible for...