Medtronic launching a new frontier in treating high blood pressure
FDA approves medical device-related hypertension therapies in the U.S.
For the first time ever, there are now approved medical device-related procedures to treat Americans with hypertension (high blood pressure).
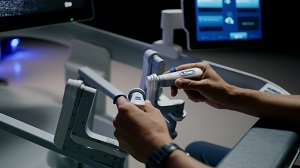
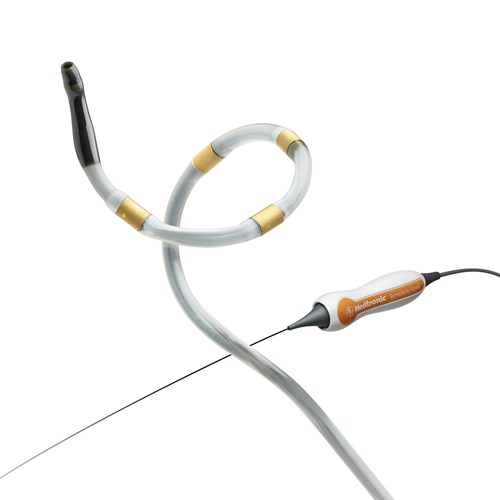
The U.S. Food and Drug Administration (FDA) today approved the Medtronic Symplicity Spyral™ renal denervation system for use in the United States. The system, which carries the FDA’s Breakthrough Device designation, uses radio frequency to disrupt overactive nerves in the kidneys and help lower blood pressure.
“This launches a new frontier in the fight against high blood pressure,” said Jason Weidman, president of the Coronary and Renal Denervation business at Medtronic. “Especially for the many patients whose hypertension doesn’t respond to drugs, or for people who struggle to keep up with their drug regimen, Symplicity Spyral has the potential to make a major difference in their lives.”
Half of all Americans are believed to have high blood pressure but about 75 percent of them don’t have it under control.1 Until now, the only effective means of treating high blood pressure has been through lifestyle changes and/or drug therapy.
FDA approval of renal denervation gives those patients, and their doctors, another option.

“In combination with medication and lifestyle changes, renal denervation opens the door to manage high blood pressure in the long term through a safe and effective procedure.2,3,4,5 It’s absolutely exciting to think about how this complementary therapy can help treat the problem of high blood pressure for many more people,” said Dr. David Kandzari, Chief, Piedmont Heart Institute, and Chief Scientific Officer, Piedmont Healthcare in Atlanta, Georgia.
Researchers have long known that the sympathetic nervous system, which connects the brain, heart, and kidneys, can impact blood pressure.6 In some people, the nerves connected to the kidneys experience excessive activity, causing high blood pressure.7
Renal denervation disrupts that overactivity through a minimally invasive procedure. The doctor inserts a thin tube into an artery in the leg and navigates it into the kidneys. Once there, the doctor uses precisely tuned radio frequency energy to ablate nerves surrounding the exterior of the renal arteries, without damaging the arteries themselves. The overactivity between the brain and kidneys is disrupted, and studies show significant reductions in blood pressure often follow soon after. 8,9,10
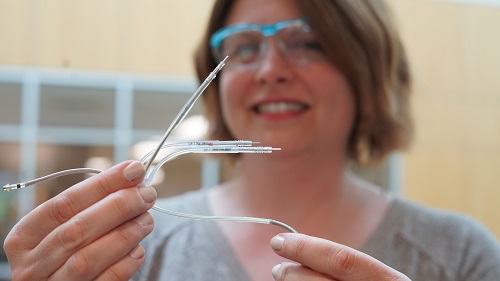
“Renal denervation has been proven safe, and data over several years indicates its effects sustained11,” said Dr. Stefan Tunev, a senior distinguished scientist at Medtronic who has studied renal denervation for years. “It’s incredibly exciting to think that we now have an additional tool to fight high blood pressure, which is one of the largest inadequately addressed medical issues of our time.”
In 2021 alone, hypertension was a primary or contributing cause of 691,095 deaths in the United States.12 And hypertension costs the U.S. healthcare system more than $130 billion in direct costs every year, according to the Partnership to Advance Cardiovascular Health.
Renal denervation offers new hope for people like 68-year-old Paul Griffin of Atlanta, Georgia. He knows the battle with high blood pressure all too well.
Peace of mind
Griffin suffered from high blood pressure for nearly 30 years. Diagnosed in his 40s, he found that drugs kept his blood pressure in check for several years. But eventually, doctors had to add more and more medication to his daily regimen to control his blood pressure. Some had side effects, and ultimately drug therapy alone did not get his blood pressure down far enough to satisfy Griffin’s doctor.

“I was taking four medications at once, and at its best, my blood pressure was still uncontrolled,” Griffin said. “Something else had to be done.”
He learned about a clinical study for the Medtronic Symplicity Spyral renal denervation system through Facebook. He did his own research, consulted his doctor, and applied to enroll in the study.
“My blood pressure improved almost immediately after the procedure,” Griffin said. “I'm seeing a reduction in my blood pressure and I'm feeling more vibrant and energetic. And I rest easier every night feeling like we finally have this under control.” †
Renal denervation alone may not eliminate a patient’s need for drug therapy, but it can help reduce blood pressure without adding another pill to a patient’s daily regimen.13,14,15
Breakthrough technology
Symplicity Spyral received the FDA’s breakthrough device designation in 2020. The designation is intended to help patients gain more timely access to medical devices that have the potential to make a significant impact in the diagnosis or treatment of life-threatening conditions.

“The term breakthrough represents a therapy unlike any other that has existed in the past,” said Dr. Kandzari. “Renal denervation is proven safe and it’s proven to reduce blood pressure for many patients. This truly represents breakthrough technology that is offering a therapeutic alternative to many patients.”
FDA approval of the Symplicity Spyral renal denervation system is the culmination of a 14-year research and development program at Medtronic. Manufacturing of the device for U.S. distribution is already underway, and Medtronic hopes to make it widely available soon to physicians and healthcare systems.
“Symplicity Spyral is a shining example of Medtronic at its best,” said Sean Salmon, executive vice president and president of the Medtronic cardiovascular portfolio. “We bring science, engineering, and technology together to solve really difficult health problems. If it takes years to find solutions, that’s what we’ll do. This device has the potential to help a lot of people live healthier lives. That’s why we’re here. It’s very gratifying and we’re anxious to get started.”
†Results may vary. Not all patients may see a drop in blood pressure. Talk to your doctor to learn more about the procedure.
Sources:
1 Vital Signs: Awareness and Treatment of Uncontrolled Hypertension Among Adults – United States, 2003-2010. Morbidity and MMWR. CDC. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6135a3.htm. Accessed September 28, 2023.
2 Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham controlled trial. Lancet. May 2, 2020;395(10234):1444-1451.
3 Kandzari DE. Renal denervation in the presence of anti-hypertensive medications: six-month results from the randomized, blinded, sham-controlled SPYRAL HTN-ON MED trial. Presented at AHA November, 2022.
4 Mahfoud F, Mancia G, Schmieder R, et al. Renal Denervation in high-risk patients with hypertension. J Am Coll Cardiol. June 16, 2020;75(23):2879-2888.
5 Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. April 9, 2022;399(10333):1401-1410.
6 American Heart Association. How High Blood Pressure Can Lead to Kidney Damage or Failure. https://www.heart.org/en/health-topics/high-blood-pressure/health-threats-from-high-blood-pressure/how-high-blood-pressure-can-lead-to-kidney-damage-or-failure
7 Sata Y, Head G, Denton K, et al. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Frontiers in Medicine, 2018.
8 Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. The Lancet. 2018 Jun 9;391(10137):2346-2355.
9 Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomized, sham-controlled trial. Lancet. May 2, 2020;395(10234):1444-1451.
10 Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. The Lancet. 2017;390:2160–2170.
11 Medtronic data on file. Global Symplicity Registry clinical data snap, March 2023.
12 Facts about Hypertension. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/bloodpressure/facts.htm. Accessed August 10, 2023.
13 Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. The Lancet. 2018 Jun 9;391(10137):2346-2355.
14 Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomized, sham-controlled trial. The Lancet 2020; Published online March 29, 2020. DOI: 10.1016/S0140-6736(20)30554-7.
15 Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. The Lancet. 2017;390:2160–2170.
Important Safety Information
The Symplicity™ blood pressure procedure (BPP) is a minimally invasive procedure approved to help lower high blood pressure. The procedure is approved as a complement to treatments you may already be trying, such as lifestyle modifications and high blood pressure medications that might not be adequately controlling your blood pressure.
Receiving the Symplicity BPP should be a based on a joint decision between you and your doctor consider the benefits and risks of the device and procedure. Please talk to your doctor to decide whether or not the Symplicity BPP is right for you.
If you have a pacemaker or an ICD your doctor will follow-up with steps to take ahead of the procedure if you decide it is right for you.
At the time of your procedure, your doctor may detect certain anatomical conditions (e.g., your blood vessels are too big or too small) that do not allow the blood pressure procedure to continue.
You should not receive the procedure if you cannot tolerate medications that are required for the procedure, like atropine, nitroglycerin, systemic blood thinners, or certain pain medications. These medications are to help you in case your heart rate drops too low, you experience pain, or your blood vessels tighten during the procedure. You should not receive the procedure if you are pregnant.
The Symplicity BPP has not been studied in patients:
- Who are breastfeeding
- Who are under 18 years old
- Who have isolated systolic hypertension (only the “top number” of your blood pressure is high)
- Who have secondary causes of high blood pressure
- Who have had a renal stent placed less than 3 months prior to the procedure
- Who had a prior minimally invasive treatment in their renal arteries (stenting, angioplasty or prior renal denervation)
Potential Risks of the Symplicity BPP: (Note that you may experience other problems that have not been previously observed with this procedure)
- Allergic reaction to the imaging solution
- Damage to your arteries
- Future narrowing of your arteries
- Arterio-enteric fistula (an abnormal connection between your aorta and your gastrointestinal tract)
- AV fistula (an irregular connection between an artery and a vein)
- Bleeding or blood clots
- Bruising where the device enters your body (mild or severe)
- Cardiac arrest or Heart attack
- Death
- Deep vein thrombosis
- Swelling
- Slow heart rate
- Infection
- Low or high blood pressure
- Damage to your kidneys that may cause one or both to stop working
- Nausea or vomiting
- Peripheral ischemia (lack of blood supply to your limbs)
- Pulmonary embolism (a sudden block in your arteries that send blood to your lungs)
- Pseudoaneurysm (blood collecting on the outside of a vessel wall causing a balloon-like widening)
- Pain or discomfort
- Skin burns from the failure of the equipment during the procedure
- Exposure to radiation
- Stroke
For further information, please call and/or consult Medtronic at 707-525-0111 or Medtronic’s website at medtronic.com
L001-11132023
Related content