Open-Heart Surgery, Without the Open-Heart Part
FDA Approves Harmony Transcatheter Pulmonary Valve.
March 26, 2021: The U.S. Food and Drug Administration (FDA) today approved a first-of-its-kind heart valve that does something extraordinary. It takes the “open-heart” part out of certain open-heart surgeries.

20-year-old Jack Hurley couldn’t be happier.
"One of the best things about the Harmony valve was not what it did for me, but for what I didn't have to do, like spend lots of time in the hospital with a long recovery time,” he said.
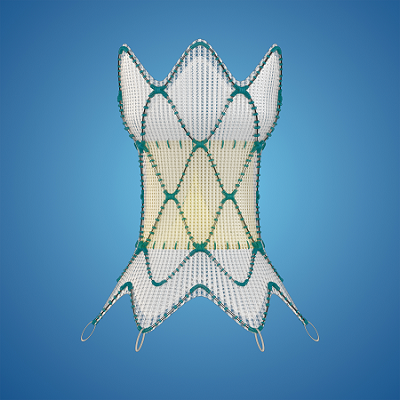
The Medtronic Harmony™ Transcatheter Pulmonary Valve (TPV) is the first pulmonary heart valve in the world to be approved for patients with a specific type of congenital heart disease without requiring open-heart surgery. Rather than cut open a patient’s chest to repair the heart, surgeons implant Harmony TPV through a much less invasive approach. They load the valve onto a catheter, make a small incision in the femoral vein or neck and deliver the valve directly inside the heart. Jack went home the next day and felt like he was back to normal within a week.

“We are always looking for less invasive ways to treat our pediatric patients, and this new device will allow us to avoid open-heart surgery in many cases,” said Matthew J. Gillespie, M.D., attending interventional cardiologist, Director of the Cardiac Catheterization Laboratory, Co-Director of the Pediatric Valve Center at Children's Hospital of Philadelphia, and an investigator in the Harmony Pivotal Trial. Dr. Gillespie implanted Jack’s Harmony valve. “For most patients, we are setting them up for a healthier life associated with their congenital heart disease.”
Jack was among an estimated 40,000 babies born every year in the U.S. with congenital heart disease (CHD).1 Jack’s pulmonary heart valve, which allows blood to leave the heart and get oxygen from the lungs, didn’t work properly. Surgeons repaired it when Jack was just three months old. But his family always knew the fix was temporary. The time would come, probably in Jack’s late teens or 20’s, that he would need open-heart surgery again.
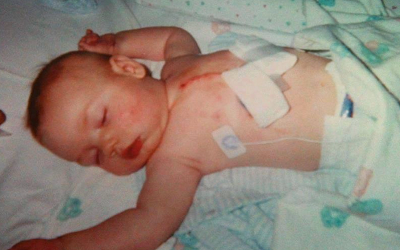
“The idea that he needed another open-heart surgery at 18 years old was just devastating to me,” said Jack’s mom Colleen. “It was such a relief for us to have a minimally-invasive option. And to see him back to living a normal life so quickly. It’s just amazing.”
Babies like Jack, born with chronic heart disease, face the possibility of multiple open-heart surgeries over the course of their lives. It can take patients weeks to recover from open-heart surgery, and each subsequent surgical procedure carries more risk than the previous one. An estimated 1.6 million adults currently live with CHD.2 Thousands of them may now benefit from Harmony TPV.

“It’s our Mission to bring life-improving therapies to as many people as possible,” said Nina Goodheart, president of the Structural Heart & Aortic operating unit at Medtronic. “Harmony does exactly that. Procedures are shorter and far less traumatic for patients. They spend less time in the hospital and recover faster. It’s very gratifying for us to be able to provide this option to CHD patients and their families.”
“Being able to offer this therapy will increase the options available to patients and families and decrease the amount of time patients spend in the hospital and in recovery,” added Dr. Gillespie.
Harmony is among several Medtronic devices that received the FDA’s Breakthrough Device Designation, a unique federal effort intended to speed up the approval process of certain life-saving technologies. Harmony is the third Medtronic device to receive the designation and then receive FDA approval.
“The strong collaboration among physicians, Medtronic and the FDA, from the earliest study phase all the way to approval, helped bring Harmony TPV to patients much faster than it might have otherwise,” Goodheart said.
Jack and his family are grateful. He’s active in sports, working part-time as a landscaper, and studying engineering in school.
“Life is the best it could be for me right now,” he said. “Whatever I do, I want to help people. I’ve been on the receiving end. I know how that feels. It would be great to be on the giving end someday.”
L001-03092021
NEXT: Getting the latest med tech to patients faster
Improving the Lives of Two People Every Second
Read Company Overview
1 Hoffman JL, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900.
2 Adult Congenital Heart Association (ACHA).
IMPORTANT SAFETY INFORMATION
INDICATIONS The Harmony Transcatheter Pulmonary Valve (TPV) System is indicated for use in the management of pediatric and adult patients with severe pulmonary regurgitation (i.e., severe pulmonary regurgitation as determined by echocardiography and/or pulmonary regurgitant fraction ≥ 30% as determined by cardiac magnetic resonance imaging) who have a native or surgically-repaired right ventricular outflow tract and are clinically indicated for surgical pulmonary valve replacement.
CONTRAINDICATIONS The following are contraindications for the use of this device: Active bacterial endocarditis or other active infections; known intolerance to Nitinol (titanium or nickel) or an anticoagulation/antiplatelet regimen.
WARNINGS General Implantation of the Harmony TPV system should be performed only by physicians who have received Harmony TPV system training. The transcatheter pulmonary valve (TPV) is to be used only in conjunction with the Harmony delivery catheter system (DCS). This procedure should only be performed where emergency pulmonary valve surgery can be performed promptly. Do not use any of the Harmony TPV system components if any of the following has occurred: It has been dropped, damaged, or mishandled in any way or if the Use By date has elapsed
Transcatheter pulmonary valve (TPV) This device was designed for single use only. Do not reuse, reprocess, or resterilize the TPV. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death. Do not resterilize the TPV by any method. Exposure of the device and container to irradiation, steam, ethylene oxide, or other chemical sterilants renders the device unfit for use. The device is packaged with a temperature sensor. Do not freeze the device. Do not expose the device to extreme temperatures. Do not use the device if the arrow on the sensor points to the symbol that indicates that the temperature limit has been exceeded. Do not use the device if any of the following have occurred: The tamper-evident seal is broken. The serial number tag does not match the container label. The arrow on the sensor points to the symbol that indicates that the temperature limit has been exceeded. The device is not completely covered by the storage solution. Do not contact any of the Harmony TPV system components with cotton or cotton swabs. Do not expose any of the Harmony TPV system components to organic solvents, such as alcohol. Do not introduce air into the catheter. Do not expose the device to solutions other than the storage and rinse solutions. Do not add or apply antibiotics to the device, the storage solution, or the rinse solution. Do not allow the device to dry. Maintain tissue moisture with irrigation or immersion. Do not attempt to repair a damaged device. Do not handle the valve leaflet tissue or use forceps to manipulate the valve leaflet tissue. Do not attempt to recapture the device once deployment has begun. Do not attempt to retrieve the TPV if any one of the outflow TPV struts is protruding from the capsule. If any one of the outflow TPV struts has deployed from the capsule, the TPV must be released from the catheter before the catheter can be withdrawn. Do not attempt post-implant balloon dilatation (PID) of the TPV during the procedure, which may cause damage to or failure of the TPV leading to injury to the patient resulting in reintervention. Delivery Catheter System (DCS) This device was designed for single use only. Do not reuse, reprocess, or resterilize the DCS. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death. Do not reuse or resterilize the DCS. If resistance is met, do not advance the guidewire, DCS, or any other component without first determining the cause and taking remedial action. Do not remove the guidewire from the DCS at any time during the procedure.
PRECAUTIONS General Clinical long-term durability has not been established for the Harmony TPV. Evaluate the TPV performance as needed during patient follow-up. The safety and effectiveness of Harmony TPV implantation in patients with pre-existing prosthetic heart valve or prosthetic ring in any position has not been demonstrated. The Harmony TPV system has not been studied in female patients of child-bearing potential with positive pregnancy. Before Use Exposure to glutaraldehyde may cause irritation of the skin, eyes, nose, and throat. Avoid prolonged or repeated exposure to the chemical vapor. Use only with adequate ventilation. If skin contact occurs, immediately flush the affected area with water (for a minimum of 15 minutes) and seek medical attention immediately. The TPV and the glutaraldehyde storage solution are sterile. The outside of the TPV container is nonsterile and must not be placed in the sterile field. The TPV and DCS should be used only in a sterile catheterization laboratory (cath lab) environment. Ensure that sterile technique is used at all times. Strictly follow the TPV rinsing procedure. For TPV 25: Ensure that all green sutures have been removed from the attachment suture loops on the TPV before loading onto the DCS. Prevent contamination of the TPV, its storage solution, and the DCS with glove powder. Verify the orientation of the TPV before loading it onto the DCS. The inflow end of the TPV with attachment suture loops must be loaded first. Do not place excessive pressure on the TPV during loading. Inspect the sealed DCS packaging before opening. If the seal is broken or the packaging has been damaged, sterility cannot be assured. Proper functioning of the DCS depends on its integrity. Use caution when handling the DCS. Damage may result from kinking, stretching, or forceful wiping of the DCS. This DCS is not recommended to be used for pressure measurement or delivery of fluids. Carefully flush the DCS and maintain tight DCS connections to avoid the introduction of air bubbles.
During Use The TPV segment is rigid and may make navigation through vessels difficult. Do not advance any portion of the DCS under resistance. Identify the cause of resistance using fluoroscopy and take appropriate action to remedy the problem before continuing to advance the DCS. Careful management of the guidewire is recommended to avoid dislodgement of the TPV during DCS removal. Once deployment is initiated, retrieval of the TPV from the patient is not recommended. Retrieval of a partially deployed valve may cause mechanical failure of the delivery catheter system or may cause injury to the patient. Refer to section below for a list of potential adverse events associated with the Harmony TPV implantation. During deployment, the DCS can be advanced or withdrawn prior to the outflow struts protruding from the capsule. Once the TPV struts contact the anatomy during deployment, it is not recommended to reposition the device. Advancing the catheter forward once the TPV struts make contact with the anatomy may lead to an undesired deployment or may cause damage to or failure of the TPV and injury to the patient. Refer to section below for a list of potential adverse events associated with the Harmony TPV implantation. Physicians should use judgment when considering repositioning of the TPV (for example, using a snare or forceps) once deployment is complete. Repositioning the bioprosthesis is not recommended, except in cases where imminent serious harm or death is possible (for example, occlusion of the main, left, or right pulmonary artery). Repositioning of a deployed valve may cause damage to or failure of the TPV and injury to the patient. Refer to section below for a list of potential adverse events associated with the Harmony TPV implantation. Ensure the capsule is closed before DCS removal. If increased resistance is encountered when removing the DCS through the introducer sheath, do not force passage. Increased resistance may indicate a problem and forced passage may result in damage to the device and harm to the patient. If the cause of resistance cannot be determined or corrected, remove the DCS and introducer sheath as a single unit over the guidewire, and inspect the DCS and confirm that it is complete. If there is a risk of coronary artery compression, assess the risk and take the necessary precautions. Endocarditis is a potential adverse event associated with all bioprosthetic valves. Patients should make their health care providers aware that they have a bioprosthetic valve before any procedure. Postprocedure, administer appropriate antibiotic prophylaxis as needed for patients at risk for prosthetic valve infection and endocarditis. Prophylactic antibiotic therapy is recommended for patients receiving a TPV before undergoing dental procedures. Postprocedure, administer anticoagulation and/or antiplatelet therapy per physician/clinical judgment and/or institutional protocol. Excessive contrast media may cause renal failure. Preprocedure, measure the patient’s creatinine level. During the procedure, monitor contrast media usage. Conduct the procedure under fluoroscopy. Fluoroscopic procedures are associated with the risk of radiation damage to the skin, which may be painful, disfiguring, and long-term.
POTENTIAL ADVERSE EVENTS Potential risks associated with the implantation of the Harmony TPV may include, but are not limited to, the following:
- death
- valve dysfunction
- tissue deterioration
- hematoma
- heart failure
- cerebrovascular incident
- perforation
- rupture of the right ventricular outflow tract (RVOT)
- compression of the aortic root
- compression of the coronary arteries
- sepsis
- pseudoaneurysm
- erosion
- stent fracture
- arrhythmias
- device embolization or migration
- pulmonary embolism
- occlusion of a pulmonary artery
- laceration or rupture of blood vessels
- device misorientation or misplacement
- valve deterioration
- regurgitation through an incompetent valve
- physical or chemical implant deterioration
- paravalvular leak
- valve dysfunction leading to hemodynamic compromise
- residual or increasing transvalvular gradients
- progressive stenosis and obstruction of the implant
- hemorrhage
- endocarditis
- thromboembolism
- thrombosis
- thrombus
- intrinsic and extrinsic calcification
- bleeding
- bleeding diathesis due to anticoagulant use
- fever
- pain at the catheterization site
- allergic reaction to contrast agents
- infection
- progressive pulmonary hypertension
- progressive neointimal thickening and peeling
- leaflet thickening
- hemolysis
- General surgical risks applicable to transcatheter pulmonary valve implantation:
- abnormal lab values (including electrolyte imbalance and elevated creatinine)
- allergic reaction to antiplatelet agents, contrast medium, or anesthesia
- exposure to radiation through fluoroscopy and angiography
- permanent disability.
Please reference the Harmony TPV system Instructions for Use for more information regarding indications, warnings, precautions, and potential adverse events.
Caution: Federal Law (USA) restricts these devices to the sale by or on the order of a physician.
