Why one family hopes for a ‘smarter’ device
Working toward future technology that could bring peace of mind to Hydrocephalus patients and their families
Jen and Brian Martinage were just hours from taking their newborn son, Patrick, home from the hospital when he suddenly had a seizure that led to the discovery of a brain hemorrhage.
At just two days old, Patrick needed brain surgery.
“Within minutes our world turned upside down,” Jen Martinage said.
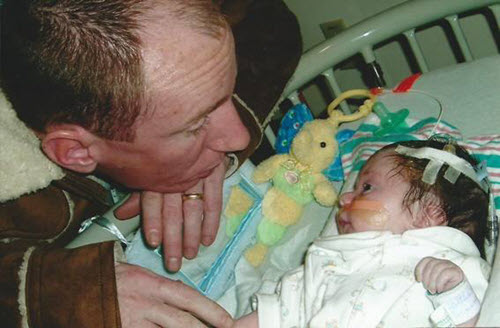
Over the next six weeks, doctors discovered Patrick’s brain was not absorbing cerebrospinal fluid the way it should — a condition called hydrocephalus for which there is currently no cure.
The only healthcare technology treatment available involves a drain system, called a shunt, that helps remove the abnormal build-up of cerebrospinal fluid that puts pressure on the brain.
Without it, Patrick could die.
As part of the shunt system, a catheter drains the fluid from the brain through the shunt valve implanted just behind Patrick’s ear. Another catheter attached to the bottom of the valve then discharges the fluid into his abdomen area where it is naturally absorbed by his body. Patrick now has a Medtronic shunt system, called Strata™ II, keeping him alive.
Thirteen brain surgeries
There has been innovation in shunt technology in recent years. Our Strata™ II device, for instance, helps prevent over-drainage or under-drainage of fluid, and our new StrataMR™ II,
slated for limited release next year, is more resistant to strong magnetic fields.
“Shunts are pretty complex systems,” said Martinage. “Unfortunately, they are not perfected yet.”
Over time, the catheters can get blocked as cells in the brain attack the foreign object and attach themselves — prompting emergency surgery to replace the affected parts. In the sixteen years of his life, Patrick has already endured 13 brain surgeries to revise or replace parts of his shunt system.

Patrick isn’t alone. Hydrocephalus is the number one reason for brain surgery in children, and many patients undergo multiple revision surgeries in their lifetime.
A smarter shunt
Jen and Brian, like other parents of hydrocephalus patients, are glad to see improvement in the technology. Ultimately though, they’re hoping the future brings a ”smart shunt” — a device that would connect to a smart phone to monitor the flow of fluid and alert them if there is a problem. Currently, parents like Jen and Brian don’t know there’s a problem until their child shows symptoms of shunt failure. Those symptoms can range from a headache to seizures.
“It doesn’t have to be this way,” said Jen Gemkow, a program manager in Medtronic Enabling Technologies who supports shunt product development. “There’s so much uncertainty in the therapy today and ultimately, we want to bring peace of mind to our patients and their caregivers. We think we can do that, by giving them or their clinicians access to data from their device that could help in the early detection of problems. We’re closely examining how these ‘smart’ shunts could be part of our future therapies.”
The Neurosciences team is closely following the latest in smart shunt research and learning from other operating units that have already incorporated smart technology into their devices.
For now, parents like Jen and Brian, who are active in the Hydrocephalus Association, are doing all they can to help raise money to fund research for the condition while they wait for more innovation.
“Until a cure is found, we need better treatments,” said Jen Martinage. “Brain surgery should not be the only treatment option for hydrocephalus.”
L001-10252022
Strata II ™ Valve Brief Summary
Indications The Strata II valve is a shunt component designed to provide continued cerebrospinal fluid (CSF) flow from the ventricles of the brain into the right atrium of the heart or the peritoneal cavity. The Strata II valve allows the physician to non-invasively adjust the pressure/flow performance level pre- and post-implantation without the need for radiographic confirmation in order to address changing patient needs. Contraindications Shunting of CSF into the peritoneal cavity or other areas of the body should not be carried out if there is infection in any areas in which the various components of the shunt system will be implanted. These include infections of the scalp and other skin area through which the shunt system will traverse, the meninges and cerebral ventricles, peritoneum and intraperitoneal and retroperitoneal organs, pleura and blood stream. CSF shunting is contraindicated if there is infection present in any area of the body. Additionally, shunting into the atrium of patients with congenital heart disease or other serious cardiopulmonary abnormalities is contraindicated. Warnings and Precautions Refer to product package insert for instructions, warnings, precautions and complications. This literature is intended for the exclusive use of physicians. Rx only.
UC202306649EN
