| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Feb 3, 2026
Deal demonstrates Medtronic's commitment to expanding pipeline through strategic investments and targeted acquisitions Exercising option to acquire will bolster Medtronic's interventional...
-
Jan 23, 2026
First-of-its-kind, rotation-free single-shot PFA catheter supported by strong safety and efficacy data adds to the groundbreaking Affera family of technologies in Europe Successful completion of...
-
Oct 26, 2025
Long-term data shows significant and sustained blood pressure reductions in both office-based and 24-hour ambulatory blood pressure, reinforcing the durability, safety and effectiveness of the...
-
Sep 15, 2025
Study to evaluate whether a new approach to pacing the heart can improve the lives of patients with heart failure with preserved ejection fraction (HFpEF) who have limited treatment options today...
-
May 7, 2025
Star Jones, award winning television personality & women's heart health advocate, helps kick off heart health conversations this Mother's Day with the Medtronic 'Letter to My Mother' campaign GALWAY,
-
Apr 26, 2025
One-year clinical trial data for the next-generation, investigational, Sphere-360™ single-shot PFA catheter show impressive safety, performance, and efficiency results for paroxysmal Afib...
-
Apr 25, 2025
Adding to the Medtronic portfolio of catheter-based lead solutions, the novel OmniaSecure defibrillation lead allows for precise delivery and placement in the right ventricle Heart Rhythm 2025:...
-
Mar 9, 2025
CRT 2025 Late Breaking Science features largest head-to-head randomized control TAVR trial to primarily enroll women using the two most widely used global TAVR devices GALWAY, Ireland and WASHINGTON,
-
Jan 13, 2025
National coverage analysis is the first-of-its kind for a minimally invasive, interventional treatment for high blood pressure Milestone is supported by large public health need and robust,...
-
Oct 24, 2024
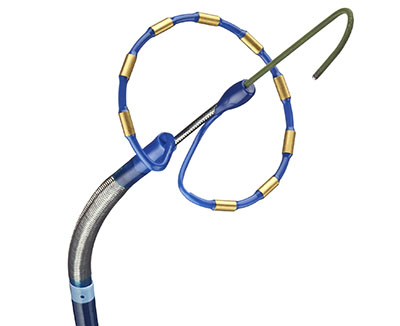
First-of-its-kind, all-in-one HD-mapping and dual energy (pulsed field and radiofrequency) ablation catheter Highly anticipated by electrophysiologists for its innovation and demonstrated safety...
-
Sep 27, 2024
APHRS: New data confirm robust long-term durability and highly efficient procedure performed without fluoroscopy Successful launch in Japan follows recent reimbursement approval Approvals across...
-
May 17, 2024
HRS late breaking data: SPHERE Per-AF trial demonstrates novel all-in-one system delivers exceptional results, plus increased efficiency and improved quality of life DUBLIN and BOSTON, May 17,...
-
May 17, 2024
Global LEADR clinical trial meets safety and effectiveness objectives; results presented at Heart Rhythm 2024 and simultaneously published in Heart Rhythm DUBLIN and BOSTON, May 17, 2024...
-
Apr 8, 2024
EHRA late-breaking data: Results highlight efficacy, safety, and durability of the novel PFA catheter that is fully integrated with Affera™ Mapping and Ablation System DUBLIN and BERLIN, April...
-
Apr 7, 2024
New data from largest head-to-head randomized control TAVR trial demonstrates non-inferior clinical outcomes and superior valve performance for Evolut TAVR compared to Sapien™ at one year DUBLIN and
-
Apr 7, 2024
Despite prevalence of chronic conditions associated with aortic stenosis in women over 65, the majority have never been referred to a cardiologist; Racial disparities may present further...
-
Mar 27, 2024
The Evolut™ FX+ TAVR system leverages market-leading valve performance with addition of larger windows to facilitate coronary access DUBLIN, March 27, 2024 /PRNewswire/ -- Medtronic plc (NYSE:...
-
Jan 5, 2024
New pacemakers offer 40% more battery life,1 extend Medtronic legacy of pacing leadership DUBLIN, Jan. 5, 2024 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology,...
-
Dec 13, 2023
Safe, efficient, and effective treatment for both paroxysmal and persistent atrial fibrillation DUBLIN, Dec. 13, 2023 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare...
-
Nov 17, 2023
The Symplicity™ blood pressure procedure offers patients a new adjunct approach to lowering blood pressure Approval is the culmination of ten years of clinical research and development of the...
-
Oct 24, 2023
TCT 2023: Medtronic adds to the body of evidence for Evolut TAVR with late-breaking clinical trial data from the Evolut Low Risk Trial DUBLIN and SAN FRANCISCO, Oct. 24, 2023 /PRNewswire/ --...
-
Oct 23, 2023
First-of-its-kind Aurora EV-ICD™ system offers single device, single procedure with lead placed outside of heart and veins DUBLIN, Oct. 23, 2023 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a...
-
May 20, 2023
Late-breaking data at Heart Rhythm 2023 underscore performance of first-of-its-kind investigational defibrillator with the lead placed under the breastbone, outside the heart and veins...
-
May 16, 2023
AccuRhythm AI algorithms now cleared by FDA for the Reveal LINQ ICM; enhancements made to the AF algorithm for the LINQ II ICM DUBLIN, May 16, 2023 /PRNewswire/ -- Medtronic plc (NYSE: MDT) today...
-
May 1, 2023
New pacemakers offer 40% more battery life,1 extend Medtronic legacy of pacing leadership DUBLIN, May 1, 2023 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology,...
-
Mar 15, 2023
First of its kind, all-in-one Sphere-9™ Catheter with pulsed field ablation, radiofrequency, and high density mapping integrated with intuitive mapping and navigation platform DUBLIN, March 15, 2023
-
Mar 6, 2023
ACC.23/WCC late-breaking data: PULSED AF, one of the most rigorously executed PFA clinical studies to date, exceeds safety and efficacy performance goals in the treatment of paroxysmal and...
-
Feb 17, 2023Medtronic Receives CE Mark for Extravascular Defibrillator System That Treats Abnormal Heart Rhythms
EV-ICD defibrillation lead placed outside of heart and veins, preserving vasculature DUBLIN, Feb. 17, 2023 /PRNewswire/ -- Medtronic plc (NYSE:MDT) has received CE (Conformité Européenne) Mark...
-
Feb 8, 2023
ISC23: Late-breaking results from STROKE AF study reinforce importance of long-term continuous cardiac monitoring in ischemic stroke patients DUBLIN and DALLAS, Feb. 8, 2023 /PRNewswire/ --...
-
Dec 5, 2022
SPHERE Per-AF will determine the safety and effectiveness of the Sphere-9 cardiac ablation and mapping catheter with the Affera mapping and navigation system DUBLIN, Dec. 5, 2022 /PRNewswire/ --...
-
Nov 7, 2022
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced the six-month results from the full cohort of the SPYRAL HTN-ON MED clinical trial. The data were presented...
-
Oct 17, 2022
Medtronic "Conduction System Pacing" Expanded Indication Now Includes Left Bundle Branch Area Pacing in Addition to His-Bundle Pacing for Patients with Slow Heart Rates DUBLIN, Oct. 17, 2022...
-
Sep 20, 2022
The LINQ II ICM is the first continuous, long-term cardiac monitor cleared by FDA for the pediatric patient population DUBLIN, Sept. 20, 2022 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global...
-
Sep 18, 2022
Long-term analysis from landmark renal denervation trial presented as Late Breaking Clinical Science, published simultaneously in The Lancet Data add to growing body of evidence supporting the...
-
Aug 30, 2022
Acquisition adds mapping and navigation platform to company's cardiac ablation portfolio and brings an integrated system of diagnostic and therapeutic tools to address a broad spectrum of arrhythmias
-
Aug 28, 2022Medtronic Extravascular ICD meets global pivotal clinical trial's safety and effectiveness endpoints
Late-breaking data presented at ESC Congress 2022 and simultaneously published in The New England Journal of Medicine confirms implant procedure safety and defibrillation success DUBLIN and BARCELONA,
-
Jul 12, 2022
Partnership underscores Medtronic's commitment to investing in innovative technologies that support physicians and patients from diagnosis to treatment CathWorks' FFRangio® System provides...
-
May 17, 2022
Medtronic adds to SPYRAL HTN Global Clinical Program with late breaking clinical data for SPYRAL HTN-ON MED and GSR-DEFINE DUBLIN and PARIS, May 17, 2022 /PRNewswire/ -- Medtronic plc (NYSE: MDT),...
-
May 13, 2022
The Onyx Frontier™ drug-eluting stent offers an innovative delivery system and builds upon the acute performance and clinical data from the Resolute Onyx™ drug-eluting stent DUBLIN, May 13, 2022 /
-
Apr 4, 2022
Medtronic adds to its robust clinical program with long-term data demonstrating the continued blood pressure lowering effect of the Symplicity renal denervation procedure Medtronic also completes...
-
Feb 18, 2022Family of focal ablation catheters are the first and only to treat specific type of supraventricular tachycardia (SVT)
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced that the Freezor™ and Freezor™ Xtra Cardiac Cryoablation Catheters are approved by the U.S. Food and Drug...
-
Jan 10, 2022World's smallest pacemaker can now treat patients with AV block, bringing miniaturized, leadless pacing to more patients
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced it has received approval from Japan's Ministry of Health, Labor and Welfare for the sale and reimbursement of...
-
Jan 10, 2022
First Medtronic TAVR system approved in China for patients with symptomatic severe aortic stenosis DUBLIN, Jan. 10, 2022 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in healthcare...
-
Jan 10, 2022
Acquisition to expand company's cardiac ablation portfolio, including first-time entry into mapping and navigation, within one of the fastest growing medtech markets DUBLIN, Jan. 10, 2022...
-
Nov 6, 2021
Transfemoral access added into landmark APOLLO Pivotal Trial following IDE approval from FDA DUBLIN and ORLANDO, Fla., Nov. 6, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in...
-
Nov 5, 2021
TCT 2021: Intermediate risk TAVR patients demonstrate strong hemodynamics and stable valve performance at five years DUBLIN and ORLANDO, Fla., Nov. 5, 2021 /PRNewswire/ -- Medtronic plc...
-
Nov 4, 2021
New data from Medtronic Patient Preference study presented at TCT 2021; Medtronic adds to its clinical leadership for renal denervation with launch of the SPYRAL AFFIRM study DUBLIN and ORLANDO,...
-
Oct 4, 2021Study Published in JAMA Cardiology Demonstrates a Three-Fold Increased Risk of Ischemic Stroke Within 30 Days of a Multi-Hour AF Event
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced the publication of a study demonstrating, through the use of a Medtronic continuous rhythm monitoring device,...
-
Sep 13, 2021Aortic Stenosis Pilot Program with Mpirik Also Underway
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced a pilot program with Mpirik to address disparities in care associated with the prevention of sudden cardiac...
-
Aug 27, 2021
Results Presented at ESC 2021 Show Micra TPS Compares Favorably to Traditional Pacemakers DUBLIN, Aug. 27, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in medical technology,...
-
Aug 24, 2021Evolut™ FX TAVR System Adds Innovative Features to Enhance Ease-of-Use and Predictable Valve Deployment
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced U.S. Food and Drug Administration (FDA) approval of its newest-generation, self-expanding transcatheter aortic...
-
Jul 28, 2021
AI Algorithms Enhance LINQ II™ Insertable Cardiac Monitor Diagnostic Accuracy for Improved Management of Patients DUBLIN, July 28, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global...
-
Jul 26, 2021
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced new data from the landmark WRAP-IT study published in Heart Rhythm, demonstrating a significantly lower...
-
Jul 26, 2021
Medtronic Prevail DCB™ Designed to Treat Complex Lesions with Superior Deliverability, Rapid Absorption of Paclitaxel DUBLIN, July 26, 2021 /PRNewswire/ -- Medtronic plc (NYSE: MDT), the global...
-
Jun 29, 2021
DEFINE AFib Study Will Leverage Data from Medtronic Insertable Cardiac Monitors and Medtronic Discovery App™ with the Goal of Informing Atrial Fibrillation (AF) Management Strategies DUBLIN,...
-
Jun 21, 2021
- First and Only Approval to Indicate Cryoablation as an Initial Rhythm Control Strategy - Offers Safe, Effective Alternative to Antiarrhythmic Drug Therapy for the Prevention of Atrial Arrythmia...
-
Jun 7, 2021Publication in JAMA Shows Reveal LINQ™ ICM Demonstrated a Seven-Fold Increase in Detecting AF vs. Standard of Care
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced clinical trial results from the STROKE AF trial demonstrating the superiority of the Reveal LINQ™ Insertable...
-
Jun 3, 2021
- Company Developing Support Program for Current HVAD Patients - Medtronic Coordinating with Global Regulators to Assure Patient Access to Left Ventricular Assist Devices DUBLIN, June 3, 2021...
-
May 18, 2021EuroPCR: Low Risk Study Demonstrates Hemodynamic Advantages of Evolut™ Transcatheter Aortic Valve Replacement (TAVR) System Over Open Heart Surgery Out to Two Years
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the complete two-year outcomes from the landmark Evolut Low Risk Trial comparing the minimally invasive Evolut™...
-
May 18, 2021
Medtronic Launches New GSR-DEFINE Study to Expand Real-World Data to 5,000 Patients DUBLIN, May 18, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today...
-
May 14, 2021Symposium During the American College of Cardiology's 70th Annual Scientific Session Offers New Real-World Insights to Advance Physician/Patient Dialogue
New findings from an American College of Cardiology's (ACC) survey revealed more than half of cardiologists (57%) believe patient preparation may lead to better quality discussions around...
-
May 3, 2021
As TAVI Patient Population Grows, PRO+ TAVI System Launches with Four Valve Sizes and Lowest Delivery Profile DUBLIN, May 3, 2021 /PRNewswire/ -- Medtronic plc (NYSE: MDT), the global leader in...
-
Apr 28, 2021
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced results from an interim analysis of the first 171 patients (including 71 roll-ins) treated in the OPTIMIZE PRO...
-
Apr 20, 2021
IN.PACT AV Access Trial 24 Month Results Presented as a Podium First at Charing Cross Symposium DUBLIN and LONDON, April 20, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in...
-
Mar 26, 2021Harmony™ Transcatheter Pulmonary Valve is First Minimally Invasive Alternative for Patients with Severe Pulmonary Regurgitation Who Have a Native or Surgically-Repaired Right Ventricular Outflow Tract
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) approval for its Harmony™ Transcatheter Pulmonary Valve...
-
Mar 4, 2021
Study Will Determine the Safety and Effectiveness of Pulsed Electrical Fields to Interrupt Irregular Heart Rhythms DUBLIN, March 4, 2021 /PRNewswire/ -- Medtronic plc (NYSE: MDT), the global...
-
Mar 2, 2021JACC: Clinical Electrophysiology: Findings Highlight Need to Better Classify Heart Failure Outcomes
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced new results from the landmark REVERSE trial, evaluating outcomes of cardiac resynchronization therapy (CRT) for...
-
Feb 17, 2021
Medtronic plc (NYSE:MDT), the global leader in medical technology, has voluntarily issued a global recall of unused Medtronic Valiant Navion™ thoracic stent graft system and informed physicians...
-
Jan 29, 2021Clinical Trial Demonstrates Procedural Efficiencies, Safety, Effectiveness and Non-Inferiority of DiamondTemp System
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) approval of the DiamondTemp™ Ablation (DTA) system...
-
Nov 16, 2020Results from Three Studies Show Medtronic Cryoablation is Superior to Drug Therapy for First-Line Treatment
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced that the primary results of the Medtronic-sponsored STOP AF First clinical trial have been published in the New...
-
Oct 26, 2020Abre™ Venous Self-Expanding Stent System Safe, Effective in Treating Challenging Deep Venous Lesions
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) approval for the Abre™ venous self-expanding stent...
-
Oct 18, 2020
Feasibility Study of Medtronic Drug-Coated Balloon Technology for Challenging Below-the-Knee Disease Shows Promise in Patients with Critical Limb Ischemia DUBLIN, Oct. 18, 2020 /PRNewswire/ --...
-
Oct 14, 2020
Half Moon Medical's Technology Now Ready for Evaluation in Early Feasibility Study DUBLIN, Oct. 14, 2020 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today...
-
Oct 14, 2020
Medtronic Also Begins Feasibility Study of Evolut TAVR System in Moderate and Asymptomatic Aortic Stenosis Patients DUBLIN, Oct. 14, 2020 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader...
-
Oct 1, 2020
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) approval for new one-month of dual-antiplatelet therapy...
-
Sep 30, 2020
Avenu's Percutaneous Fistula Creation Technology Will Strengthen the Medtronic Peripheral Vascular Access Portfolio and Reinforce Medtronic's Commitment to Improving Outcomes for Patients with...
-
Sep 23, 2020
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced first enrollments in the ALLEVIATE-HF clinical trial which will evaluate the ability of its Reveal LINQ™...
-
Sep 22, 2020
Medtronic plc (NYSE:MDT), the global leader in medical technology, has received Breakthrough Device Designation status from the U.S. Food and Drug Administration (FDA) for the TYRX™ Absorbable...
-
Sep 16, 2020Economic Analysis of the WRAP-IT Patient Population in Circulation: Arrhythmia and Electrophysiology
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced new data from the landmark WRAP-IT study, showing the TYRX™ Absorbable Antibacterial Envelope (TYRX Envelope)...
-
Sep 9, 2020
Medtronic plc (NYSE:MDT), a global leader in structural heart therapies, today announced U.S. Food and Drug Administration (FDA) approval of an early feasibility study (EFS) of the Intrepid™...
-
Aug 29, 2020ESC Congress: New Research Highlights First-Line Use of Cryoablation as Highly Effective, and with Improved Quality of Life Scores
DUBLIN, Aug. 29, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced clinical trial results demonstrating superiority of the Arctic Front™...
-
Aug 21, 2020Revised Commercial Labeling Broadens Patient Population Who May be Candidates for Transcatheter Aortic Valve Replacement (TAVR)
DUBLIN, Aug. 21, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the U.S. Food and Drug Administration (FDA) has approved revised...
-
Aug 19, 2020Results Showed IN. PACT™ AV DCB Is Safe, Reduces Reinterventions, and Helps Maintain Dialysis Access for Those Living with End-Stage Renal Disease
DUBLIN, Aug. 19, 2020 (GLOBE NEWSWIRE) -- Medtronic announced today the publication of the primary endpoint results from the IN.PACT AV Access trial in The New England Journal of Medicine.1 The...
-
Jul 7, 2020Next Generation ICM Offers Remote Programming with Improved Longevity and Enhanced Accuracy
DUBLIN, July 07, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) clearance and...
-
Jul 6, 2020Global “DISSECT-N” Post-Market Study to Broaden Evidence Base for Safety and Effectiveness of Commercially Available Endovascular Repair Technology
DUBLIN, July 06, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the start of a prospective, observational, global, multi-center,...
-
Jun 25, 2020Regardless of the Number of Anti-Hypertensive Medications Prescribed, Majority of Patients with Highest BP Showed Significant Drops at Three Years
DUBLIN, June 25, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today reported new data from the Global SYMPLICITY Registry (GSR), which showed that...
-
Jun 22, 2020The Evolut TAVI Platform Receives New Indication for Patients with Bicuspid Aortic Valves at Extreme, High or Intermediate Risk of Surgical Mortality
DUBLIN, June 22, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced CE (Conformité Européenne) mark and European launch of the Evolut™...
-
Jun 16, 2020Medtronic Self-Expanding Venous Stent Demonstrates Favorable Outcomes in Patients with Venous Outflow Obstruction
DUBLIN and LONDON, June 16, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the first-ever results from the ABRE clinical study...
-
Jun 15, 2020World’s Smallest Pacemaker Can Now Treat Patients with AV Block, Allowing More Potential Candidates for Leadless Pacing
DUBLIN, June 15, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT) today announced it has received CE (Conformité Européenne) Mark for Micra™ AV Transcatheter Pacing System (TPS), the...
-
Jun 5, 2020
DUBLIN, June 05, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received CE (Conformité Européenne) Mark for a one-month dual...
-
May 29, 2020
DUBLIN, May 29, 2020 (GLOBE NEWSWIRE) -- During this critical time, Medtronic remains committed to our strategy of delivering critical care products ― designed and indicated for Extracorporeal...
-
May 19, 2020
DUBLIN, May 19, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced the U.S. launch of Kyphon Assist™ Directional Cannula for use with its...
-
May 18, 2020
DUBLIN, May 18, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced Jeffrey J. Popma, M.D., professor of medicine at Harvard Medical School...
-
May 15, 2020Milestone Results Presented as Late-Breaking Clinical Science at SCAI 2020
DUBLIN, May 15, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT) today announced results from two late-breaking clinical trials presented virtually at the 2020 Society for Cardiovascular...
-
May 8, 2020New Data Unveiled at Heart Rhythm 2020 Demonstrate Effectiveness of App-Based Remote Monitoring of Medtronic Cardiac Devices, Significant Reduction in Complications with Micra Leadless Pacemaker
DUBLIN, May 08, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT) today announced results from late-breaking clinical trials evaluating the MyCareLink Heart™ mobile app and the Micra®...
-
Mar 30, 2020First Study in the U.S. and Japan to Report Results on One-Month DAPT Duration in High Bleeding Risk Patients with Current-Generation DES
DUBLIN, March 30, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT) today announced positive results of the Onyx ONE Clear Study that evaluated Resolute Onyx DES in high bleeding risk (HBR)...
-
Mar 29, 2020ACC.20/WCC: Low Rates of Paravalvular Leak and High Survival Observed in Late-breaking Clinical Trial of Low-Risk Bicuspid Aortic Stenosis Patients
DUBLIN, March 29, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced late-breaking clinical data from the Low Risk Bicuspid Study assessing...
-
Mar 29, 2020ACC.20/WCC: Study Finds Significant Blood Pressure Reductions Achieved with RDN in Absence of Anti-Hypertensive Medication
DUBLIN, March 29, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced first-ever clinical data from the SPYRAL HTN-OFF MED Pivotal Trial....
-
Feb 4, 2020SPYRAL DYSTAL Study to Evaluate the Effects of RDN Using Fewer, Targeted Ablations to Achieve Meaningful Blood Pressure Reductions
DUBLIN, Feb. 04, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT) today announced it will begin enrollment in a pilot study evaluating the safety and efficacy of the Symplicity™ Spyral renal...
-
Jan 30, 2020Next Generation Technology Includes Features that Automatically Adjust to Patient Needs, and Offers Physicians Heart Failure Diagnostic Insights
DUBLIN, Jan. 30, 2020 (GLOBE NEWSWIRE) -- Medtronic plc (NYSE:MDT) today announced it has received CE (Conformité Européenne) Mark for its Cobalt™ and Crome™ portfolio of implantable...