| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Mar 15, 2023
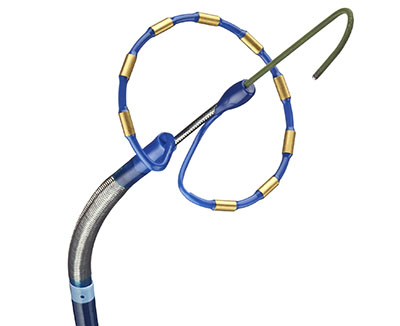
First of its kind, all-in-one Sphere-9™ Catheter with pulsed field ablation, radiofrequency, and high density mapping integrated with intuitive mapping and navigation platform DUBLIN, March 15, 2023
-
Mar 6, 2023
ACC.23/WCC late-breaking data: PULSED AF, one of the most rigorously executed PFA clinical studies to date, exceeds safety and efficacy performance goals in the treatment of paroxysmal and...
-
Feb 17, 2023Medtronic Receives CE Mark for Extravascular Defibrillator System That Treats Abnormal Heart Rhythms
EV-ICD defibrillation lead placed outside of heart and veins, preserving vasculature DUBLIN, Feb. 17, 2023 /PRNewswire/ -- Medtronic plc (NYSE:MDT) has received CE (Conformité Européenne) Mark...
-
Feb 8, 2023
ISC23: Late-breaking results from STROKE AF study reinforce importance of long-term continuous cardiac monitoring in ischemic stroke patients DUBLIN and DALLAS, Feb. 8, 2023 /PRNewswire/ --...
-
Dec 5, 2022
SPHERE Per-AF will determine the safety and effectiveness of the Sphere-9 cardiac ablation and mapping catheter with the Affera mapping and navigation system DUBLIN, Dec. 5, 2022 /PRNewswire/ --...
-
Nov 7, 2022
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced the six-month results from the full cohort of the SPYRAL HTN-ON MED clinical trial. The data were presented...
-
Oct 17, 2022
Medtronic "Conduction System Pacing" Expanded Indication Now Includes Left Bundle Branch Area Pacing in Addition to His-Bundle Pacing for Patients with Slow Heart Rates DUBLIN, Oct. 17, 2022...
-
Sep 20, 2022
The LINQ II ICM is the first continuous, long-term cardiac monitor cleared by FDA for the pediatric patient population DUBLIN, Sept. 20, 2022 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global...
-
Sep 18, 2022
Long-term analysis from landmark renal denervation trial presented as Late Breaking Clinical Science, published simultaneously in The Lancet Data add to growing body of evidence supporting the...
-
Aug 30, 2022
Acquisition adds mapping and navigation platform to company's cardiac ablation portfolio and brings an integrated system of diagnostic and therapeutic tools to address a broad spectrum of arrhythmias
-
Aug 28, 2022Medtronic Extravascular ICD meets global pivotal clinical trial's safety and effectiveness endpoints
Late-breaking data presented at ESC Congress 2022 and simultaneously published in The New England Journal of Medicine confirms implant procedure safety and defibrillation success DUBLIN and BARCELONA,
-
Jul 12, 2022
Partnership underscores Medtronic's commitment to investing in innovative technologies that support physicians and patients from diagnosis to treatment CathWorks' FFRangio® System provides...
-
May 17, 2022
Medtronic adds to SPYRAL HTN Global Clinical Program with late breaking clinical data for SPYRAL HTN-ON MED and GSR-DEFINE DUBLIN and PARIS, May 17, 2022 /PRNewswire/ -- Medtronic plc (NYSE: MDT),...
-
May 13, 2022
The Onyx Frontier™ drug-eluting stent offers an innovative delivery system and builds upon the acute performance and clinical data from the Resolute Onyx™ drug-eluting stent DUBLIN, May 13, 2022 /
-
Apr 4, 2022
Medtronic adds to its robust clinical program with long-term data demonstrating the continued blood pressure lowering effect of the Symplicity renal denervation procedure Medtronic also completes...
-
Feb 18, 2022Family of focal ablation catheters are the first and only to treat specific type of supraventricular tachycardia (SVT)
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced that the Freezor™ and Freezor™ Xtra Cardiac Cryoablation Catheters are approved by the U.S. Food and Drug...
-
Jan 10, 2022World's smallest pacemaker can now treat patients with AV block, bringing miniaturized, leadless pacing to more patients
Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced it has received approval from Japan's Ministry of Health, Labor and Welfare for the sale and reimbursement of...
-
Jan 10, 2022
First Medtronic TAVR system approved in China for patients with symptomatic severe aortic stenosis DUBLIN, Jan. 10, 2022 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in healthcare...
-
Jan 10, 2022
Acquisition to expand company's cardiac ablation portfolio, including first-time entry into mapping and navigation, within one of the fastest growing medtech markets DUBLIN, Jan. 10, 2022...
-
Nov 6, 2021
Transfemoral access added into landmark APOLLO Pivotal Trial following IDE approval from FDA DUBLIN and ORLANDO, Fla., Nov. 6, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in...
-
Nov 5, 2021
TCT 2021: Intermediate risk TAVR patients demonstrate strong hemodynamics and stable valve performance at five years DUBLIN and ORLANDO, Fla., Nov. 5, 2021 /PRNewswire/ -- Medtronic plc...
-
Nov 4, 2021
New data from Medtronic Patient Preference study presented at TCT 2021; Medtronic adds to its clinical leadership for renal denervation with launch of the SPYRAL AFFIRM study DUBLIN and ORLANDO,...
-
Oct 4, 2021Study Published in JAMA Cardiology Demonstrates a Three-Fold Increased Risk of Ischemic Stroke Within 30 Days of a Multi-Hour AF Event
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced the publication of a study demonstrating, through the use of a Medtronic continuous rhythm monitoring device,...
-
Sep 13, 2021Aortic Stenosis Pilot Program with Mpirik Also Underway
Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced a pilot program with Mpirik to address disparities in care associated with the prevention of sudden cardiac...
-
Aug 27, 2021
Results Presented at ESC 2021 Show Micra TPS Compares Favorably to Traditional Pacemakers DUBLIN, Aug. 27, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in medical technology,...