Patients Report Superior, Sustained and Profound Back Pain Relief at 12 Months with DTM™ Spinal Cord Stimulation Therapy Using the Medtronic Intellis™ Platform
Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced statistically significant 12-month results from a large, multicenter randomized controlled trial (RCT) that...
DUBLIN, Oct. 20, 2020 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced statistically significant 12-month results from a large, multicenter randomized controlled trial (RCT) that further validate the superiority of DTM™ Spinal Cord Stimulation (SCS) in providing back pain relief, compared with conventional SCS therapy, using the Medtronic Intellis™ platform.
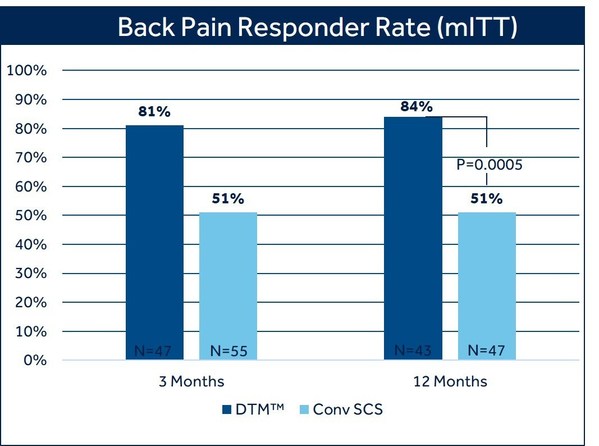
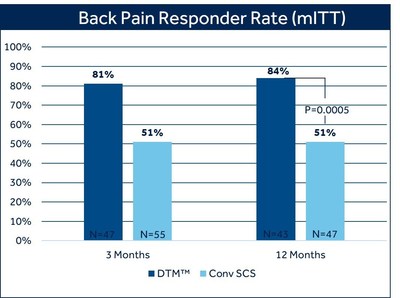
At 12 months, 84% of patients with chronic back pain treated with DTM SCS reported at least 50% pain relief, compared to 51% of patients treated with conventional SCS (p=0.0005).1 There was also a difference in the proportion of patients who reported profound back pain relief (>80% reduction in VAS score) favoring DTM SCS (69%) compared with conventional SCS (35.1%).1 The study met its primary endpoint at three months, and in pre-specified secondary analysis showed the superiority of DTM SCS compared to conventional SCS and has sustained these results at 12 months.
Pain relief was measured by the Visual Analog Scale (VAS), a widely used and accepted measure for pain intensity that records patient-reported pain levels on a scale of 0-10. Fifty-percent pain relief, as measured by VAS, is a recognized industry standard to define therapy success. The majority of DTM SCS patients in this study exceeded this threshold, with seven out of ten experiencing profound back pain relief at 12 months.
Patients treated with DTM SCS also reported an average VAS score reduction of 75% in back pain, compared with 50% treated with conventional SCS. Average VAS scores for patients treated with DTM SCS at 12 months were 1.74 for back pain and 1.45 for leg pain.1 The term remitter has previously been used to classify patients with a pain score of 2.5 or less. As a group, patients in the DTM SCS group clearly fell below this level with a mean VAS score of 1.74 for back pain and 1.4 for leg pain.
"DTM SCS has the potential to improve outcomes for patients with chronic back pain," said Michael Fishman, M.D., MBA, anesthesiologist and Interventional Pain Medicine specialist at the Center for Interventional Pain & Spine. "More than two-thirds of the patients in this RCT achieved profound pain relief of at least 80% with DTM SCS therapy. These outcomes demonstrate that DTM SCS can provide more patients profound pain relief compared with conventional SCS at 12 months, which physicians should consider when selecting a neuromodulation therapy for their patients."
"DTM SCS is based on a novel understanding of how neurons and glial cells contribute to chronic pain," said Charlie Covert, vice president and general manager of the Pain Therapies business, part of the Restorative Therapies Group at Medtronic. "The 12-month data reported demonstrate the value of Medtronic's continued focus on pursuing science-based approaches to improving human health and underscore our ability to integrate existing technologies with novel therapies like DTM SCS to improve the outcomes of people suffering from chronic pain."
Go to Medtronic.com/DTMwebinar to register for a webinar on Tues. Oct. 20 at 12 p.m. CDT to learn more about the clinical trial results.
About the Trial
The data reported are from an RCT in which SCS patients were randomized to either the treatment or control arm, with 79 subjects implanted and followed over the course of 12 months. The study previously met its primary endpoint of noninferiority compared with conventional SCS at three months, and a prespecified secondary statistical test for superiority showing the difference between DTM SCS and conventional SCS as highly significant. Medtronic previously reported three-month data from the trial in January 2020.
About DTM SCS Therapy
DTM SCS therapy is a new and proprietary spinal cord stimulation algorithm available to treat patients with chronic pain. It is delivered as a programming option via the Medtronic Intellis SCS platform. It is inspired by science and rooted in preclinical work prior to being proven in an RCT. The DTM waveform may engage a novel mechanism that modulates both neurons and glial cells, expanding the understanding of SCS mechanisms of action. Glial cells outnumber neurons in the spinal cord by 12:1 and their role in pain has been explored in research for more than 20 years.2-4
About the Intellis SCS Platform
The Intellis SCS platform, indicated to aid in the management of chronic, intractable pain, offers the world's smallest implantable neurostimulator. It is powered by proprietary Overdrive™ battery technology and was designed to overcome limitations with other SCS systems, optimized for a wide range of energy demands and provides effective long-term pain relief for patients. The neurostimulator also features SureScan™ MRI technology, allowing access to MRI anywhere in the body under certain conditions, and AdaptiveStim™ technology, which automatically adjusts stimulation based on the patient's needs and preferences in different body positions to ensure the patient receives the right dose of stimulation at the right location.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is among the world's largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
References
- Fishman M, Cordner H, et al. DTM™ SCS RCT 12-month Data Results. Presented at a Medtronic webinar, jointly supported by the North American Neuromodulation Society (NANS), World Institute of Pain(WIP), and the American Society for Pain and Neuroscience (ASPN). October 19, 2020. Webinar available on society websites.
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009 Jan;10(1):23-36.
- Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010 May-Jun;10(3):167-84.
- De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: Path to CNS centralization and chronic pain. Pain. 2006; 122:17-21.
Contacts: | |
Michelle Claypool | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-763-526-9452 | +1-763-505-4626 |
SOURCE Medtronic plc