Medtronic Reports Second Quarter Fiscal 2022 Financial Results
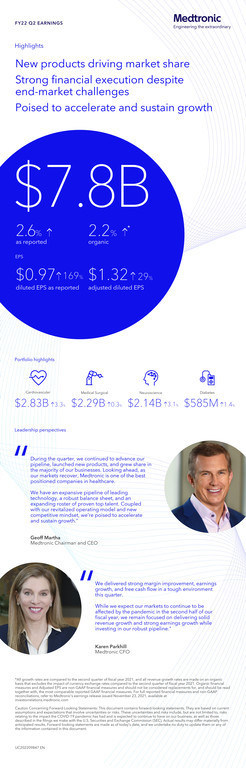
Medtronic plc (NYSE:MDT) today announced financial results for its second quarter of fiscal year 2022, which ended October 29, 2021. Key Highlights Revenue of $7.8 billion increased 3% reported...
DUBLIN, Nov. 23, 2021 /PRNewswire/ -- Medtronic plc (NYSE:MDT) today announced financial results for its second quarter of fiscal year 2022, which ended October 29, 2021.
Key Highlights
- Revenue of $7.8 billion increased 3% reported and 2% organic
- GAAP diluted EPS of $0.97; non-GAAP diluted EPS of $1.32
- Reiterates full year EPS guidance
The company reported second quarter worldwide revenue of $7.847 billion, an increase of 3% as reported and 2% on an organic basis, which excludes the $32 million benefit of foreign currency translation. Unless otherwise stated, all revenue growth rates in this press release are stated on an organic basis, which excludes the impact of foreign currency translation. The company's second quarter revenue results reflect the unfavorable market impact of COVID-19 and health system labor shortages on medical device procedure volumes, primarily in the U.S.
As reported, second quarter GAAP net income and diluted earnings per share (EPS) were $1.311 billion and $0.97, respectively, increases of 168% and 169%, respectively. As detailed in the financial schedules included at the end of this release, second quarter non-GAAP net income and non-GAAP diluted EPS were $1.792 billion and $1.32, respectively, increases of 30% and 29%, respectively.
Second quarter U.S. revenue of $3.997 billion represented 51% of company revenue and decreased 1%. Non-U.S. developed market revenue of $2.478 billion represented 32% of company revenue and increased 1% as reported and 2% organic. Emerging Markets revenue of $1.372 billion represented 17% of company revenue and increased 20% as reported and 16% organic.
"Our second quarter results reflect focused execution of our strategy and the strong underlying health of the business, despite the market impact of the pandemic resurgence and healthcare system staffing challenges on medical procedure volumes, particularly in the U.S., which affected our quarterly revenue growth," said Geoff Martha, Medtronic chairman and chief executive officer. "During the quarter, we continued to advance our pipeline, launched new products, and grew share in the majority of our businesses. Looking ahead, as our markets recover, Medtronic is one of the best positioned companies in healthcare. We have an expansive pipeline of leading technology, a robust balance sheet, and an expanding roster of proven top talent. Coupled with our revitalized operating model and new competitive mindset, we're poised to accelerate and sustain growth."
Cardiovascular Portfolio
The Cardiovascular Portfolio includes the Cardiac Rhythm & Heart Failure (CRHF), Structural Heart & Aortic (SHA), and Coronary & Peripheral Vascular (CPV) divisions. Cardiovascular revenue of $2.827 billion increased 4% as reported and 3% organic, driven by mid-single digit organic growth in CPV and low-single digit organic growth in CRHF and SHA.
- Cardiac Rhythm & Heart Failure revenue of $1.471 billion increased 3% as reported and organic. Adjusting for the discontinuation of HVAD™ System sales, CRHF revenue increased 6% organic. Cardiac Rhythm Management revenue increased in the high-single digits, driven by mid-single digit growth in Defibrillation Solutions and high-single digit growth in Cardiac Pacing Therapies, including mid-teens growth in Leadless Pacemakers on the continued global adoption of Micra™ transcatheter pacing systems. Cardiovascular Diagnostics revenue declined in the mid-single digits, as procedure volumes were affected by COVID-19 resurgence. Cardiac Ablation Solutions revenue increased in the mid-single digits on the continued adoption of Arctic Front Advance™ cryoballoon catheters and consoles.
- Structural Heart & Aortic revenue of $750 million increased 2% as reported and organic. Structural Heart grew in the high-single digits, with high-single digit growth in transcatheter aortic valves (TAVR). Cardiac Surgery increased in the high-single digits. Aortic declined in the mid-teens as a result of the previously announced global recall of the Valiant Navion™ thoracic stent graft system.
- Coronary & Peripheral Vascular revenue of $606 million increased 7% as reported and 6% organic. Coronary & Renal Denervation (CRDN) increased in the mid-single digits, driven by strength in emerging markets. Peripheral Vascular Health increased in the high-single digits, with mid-twenties endoVenous growth on strong sales of the VenaSeal™ closure system and the Abre™ venous stent.
Medical Surgical Portfolio
The Medical Surgical Portfolio includes the Surgical Innovations (SI) and the Respiratory, Gastrointestinal & Renal (RGR) divisions. Medical Surgical revenue of $2.299 billion increased 1% as reported and was flat organic, with high-single digit organic growth in SI partially offset by low double-digit organic declines in RGR. Excluding the impact of ventilator sales declines, Medical Surgical revenue increased 6% organic.
- Surgical Innovations revenue of $1.497 billion increased 7% as reported and organic. The division had mid-single digit growth in Advanced Surgical Instruments, driven by the continued adoption of the company's LigaSure™, Sonicision™, and Tri-Staple™ technologies. Hernia & Wound Management increased in the high-single digits, with strength in sutures.
- Respiratory, Gastrointestinal & Renal revenue of $802 million decreased 10% as reported and 11% organic. Excluding the impact of ventilator sales declines, RGR revenue increased 4% organic. Respiratory Interventions decreased in the mid-thirties, with sales of ventilators declining in the mid-fifties as demand returns to pre-pandemic levels. Patient Monitoring increased in the low-double digits, with mid-teens growth in the company's Nellcor™ pulse oximetry products driven in part by increased monitoring of COVID hospitalized patients. Gastrointestinal revenue increased in the mid-single digits, with low double-digit growth in Chronic & Colorectal on strength of PillCam™ system sales. Renal Care Solutions increased in the mid-single digits with low-forties growth in acute therapies driven by increased demand for adult and pediatric continuous renal replacement therapy.
Neuroscience Portfolio
The Neuroscience Portfolio includes the Cranial & Spinal Technologies (CST), Specialty Therapies, and Neuromodulation divisions. Neuroscience revenue of $2.136 billion increased 4% as reported and 3% organic, with high-single digit growth in Specialty Therapies and mid-single digit growth in Neuromodulation, partially offset by low-single digit declines in CST, all on an organic basis.
- Cranial & Spinal Technologies revenue of $1.067 billion was flat as reported and decreased 1% organic. Spine & Biologics decreased in the mid-single digits, driven by decreased spine market procedures as a result of the COVID-19 resurgence. Neurosurgery increased in the high-single digits, with strength in sales of StealthStation™ navigation systems, O-arm™ imaging systems, and Midas Rex™ powered surgical instruments.
- Specialty Therapies revenue of $634 million increased 9% as reported and 8% organic. Neurovascular increased in the low double-digits, with high-teens growth in Hemorrhagic Stroke products. Pelvic Health increased in the low-single digits, as market growth was affected by the COVID-19 resurgence. ENT grew in the low double-digits, driven by strong sales of NIM Vital™ nerve monitoring systems.
- Neuromodulation revenue of $435 million increased 6% as reported and organic. Brain Modulation increased in the mid-twenties, driven by the launch of the Percept™ PC deep brain stimulation (DBS) system and SenSight™ directional DBS lead system. Pain Therapies decreased in the mid-single digits, as high-single digit declines in Targeted Drug Delivery and flat results in Pain Stim offset high-single digit growth in Interventional.
Diabetes
Diabetes revenue of $585 million increased 2% as reported and 1% organic. Durable insulin pumps grew in the low-twenties, including high-teens growth in the U.S. and low-twenties growth in international markets on the continued launches of the MiniMed™ 770G and MiniMed™ 780G systems, respectively. Strong pump sales were offset by lower sales of consumables, which declined in the high-single digits. Sales of continuous glucose monitoring (CGM) products increased in the low-single digits.
Guidance
The company today updated its revenue growth guidance and reiterated its EPS guidance range for fiscal year 2022.
Given the greater-than-expected market impact of the pandemic and healthcare system staffing challenges in the fiscal second quarter, which is expected to continue into the second half of the fiscal year, the company now expects fiscal year 2022 revenue growth of 7-8% on an organic basis versus the prior expectation of approximately 9%. If recent foreign currency exchange rates hold, revenue growth in fiscal year 2022 would be positively affected by approximately $0 to $50 million versus the $100 to $200 million positive impact previously.
The company reiterated its fiscal year 2022 diluted non-GAAP EPS guidance range of $5.65 to $5.75, including an estimated 5 to 10 cent positive impact from foreign currency exchange based on recent rates.
"We delivered strong margin improvement, earnings growth, and free cash flow in a tough environment this quarter," said Karen Parkhill, Medtronic chief financial officer. "While we expect our markets to continue to be affected by the pandemic in the second half of our fiscal year, we remain focused on delivering solid revenue growth and strong earnings growth while investing in our robust pipeline."
Webcast Information
Medtronic will host a webcast today, November 23, at 8:00 a.m. EST (7:00 a.m. CST) to provide information about its businesses for the public, investors, analysts, and news media. This webcast can be accessed by clicking on the Investor Events link at investorrelations.medtronic.com and this earnings release will be archived at news.medtronic.com. Medtronic will be live tweeting during the webcast on its Newsroom Twitter account, @Medtronic. Within 24 hours of the webcast, a replay of the webcast and transcript of the company's prepared remarks will be available by clicking on the Investor Events link at investorrelations.medtronic.com.
Medtronic plans to report its fiscal year 2022 third and fourth quarter results on February 22, 2022, and May 26, 2022, respectively. Confirmation and additional details will be provided closer to the specific event.
Financial Schedules
The second quarter financial schedules and non-GAAP reconciliations can be viewed by clicking on the Investor Events link at investorrelations.medtronic.com. To view a printable PDF of the financial schedules and non-GAAP reconciliations, click here. To view the second quarter earnings presentation, click here.
|
MEDTRONIC PLC WORLD WIDE REVENUE(1) (Unaudited) |
||||||||||||||||||||||||||||||||||||||||||||
|
SECOND QUARTER |
SECOND QUARTER YEAR-TO-DATE(2) |
|||||||||||||||||||||||||||||||||||||||||||
|
REPORTED |
CONSTANT |
REPORTED |
CONSTANT |
|||||||||||||||||||||||||||||||||||||||||
|
(in millions) |
FY22 |
FY21 |
Growth |
Currency |
FY22 |
Growth |
FY22 |
FY21 |
Growth |
Currency |
FY22 |
Growth |
||||||||||||||||||||||||||||||||
|
Cardiovascular(3) |
$ |
2,827 |
$ |
2,725 |
3.7 |
% |
$ |
11 |
$ |
2,816 |
3.3 |
% |
$ |
5,717 |
$ |
5,158 |
10.8 |
% |
$ |
106 |
$ |
5,611 |
8.8 |
% |
||||||||||||||||||||
|
Cardiac Rhythm & Heart Failure |
1,471 |
1,426 |
3.2 |
5 |
1,466 |
2.8 |
2,954 |
2,673 |
10.5 |
51 |
2,903 |
8.6 |
||||||||||||||||||||||||||||||||
|
Structural Heart & Aortic |
750 |
733 |
2.3 |
2 |
748 |
2.0 |
1,537 |
1,360 |
13.0 |
30 |
1,507 |
10.8 |
||||||||||||||||||||||||||||||||
|
Coronary & Peripheral Vascular |
606 |
567 |
6.9 |
3 |
603 |
6.3 |
1,226 |
1,125 |
9.0 |
25 |
1,201 |
6.8 |
||||||||||||||||||||||||||||||||
|
Medical Surgical |
2,299 |
2,285 |
0.6 |
8 |
2,291 |
0.3 |
4,621 |
4,086 |
13.1 |
85 |
4,536 |
11.0 |
||||||||||||||||||||||||||||||||
|
Surgical Innovations |
1,497 |
1,393 |
7.5 |
5 |
1,492 |
7.1 |
3,051 |
2,473 |
23.4 |
59 |
2,992 |
21.0 |
||||||||||||||||||||||||||||||||
|
Respiratory, Gastrointestinal, & Renal |
802 |
893 |
(10.2) |
3 |
799 |
(10.5) |
1,570 |
1,613 |
(2.7) |
26 |
1,544 |
(4.3) |
||||||||||||||||||||||||||||||||
|
Neuroscience |
2,136 |
2,063 |
3.5 |
10 |
2,126 |
3.1 |
4,340 |
3,774 |
15.0 |
57 |
4,283 |
13.5 |
||||||||||||||||||||||||||||||||
|
Cranial & Spinal Technologies |
1,067 |
1,071 |
(0.4) |
3 |
1,064 |
(0.7) |
2,189 |
2,015 |
8.6 |
22 |
2,167 |
7.5 |
||||||||||||||||||||||||||||||||
|
Specialty Therapies |
634 |
581 |
9.1 |
6 |
628 |
8.1 |
1,275 |
1,035 |
23.2 |
25 |
1,250 |
20.8 |
||||||||||||||||||||||||||||||||
|
Neuromodulation |
435 |
411 |
5.8 |
1 |
434 |
5.6 |
875 |
725 |
20.7 |
11 |
864 |
19.2 |
||||||||||||||||||||||||||||||||
|
Diabetes |
585 |
574 |
1.9 |
3 |
582 |
1.4 |
1,157 |
1,136 |
1.8 |
29 |
1,128 |
(0.7) |
||||||||||||||||||||||||||||||||
|
TOTAL |
$ |
7,847 |
$ |
7,647 |
2.6 |
% |
$ |
32 |
$ |
7,815 |
2.2 |
% |
$ |
15,835 |
$ |
14,154 |
11.9 |
% |
$ |
277 |
$ |
15,558 |
9.9 |
% |
||||||||||||||||||||
|
(1) The data in this schedule has been intentionally rounded to the nearest million and, therefore, may not sum. |
|
(2) Fiscal year 2021 was a 53-week fiscal year, with the extra week occurring in the first fiscal month of the first quarter and included in reported prior year second quarter year-to-date results. While it is difficult to calculate the impact of the extra week, the Company estimates the extra week benefited the prior year second quarter year-to-date revenue by approximately $360 to $390 million. |
|
(3) In the fourth quarter of fiscal year 2021, the Company realigned its divisions within Cardiovascular. As a result, fiscal year 2021 results have been recast to adjust for this realignment. |
|
(4) The currency impact to revenue measures the change in revenue between current and prior year periods using constant exchange rates. |
|
MEDTRONIC PLC U.S.(1)(2) REVENUE (Unaudited) |
||||||||||||||||||||||
|
SECOND QUARTER |
SECOND QUARTER YEAR-TO-DATE |
|||||||||||||||||||||
|
REPORTED |
REPORTED |
|||||||||||||||||||||
|
(in millions) |
FY22 |
FY21 |
Growth |
FY22 |
FY21 |
Growth |
||||||||||||||||
|
Cardiovascular(3) |
$ |
1,373 |
$ |
1,377 |
(0.3) |
% |
$ |
2,793 |
$ |
2,582 |
8.2 |
% |
||||||||||
|
Cardiac Rhythm & Heart Failure |
761 |
760 |
0.1 |
1,530 |
1,431 |
6.9 |
||||||||||||||||
|
Structural Heart & Aortic |
327 |
328 |
(0.3) |
674 |
602 |
12.0 |
||||||||||||||||
|
Coronary & Peripheral Vascular |
286 |
289 |
(1.0) |
589 |
549 |
7.3 |
||||||||||||||||
|
Medical Surgical |
970 |
996 |
(2.6) |
1,959 |
1,718 |
14.0 |
||||||||||||||||
|
Surgical Innovations |
550 |
560 |
(1.8) |
1,170 |
960 |
21.9 |
||||||||||||||||
|
Respiratory, Gastrointestinal, & Renal |
420 |
436 |
(3.7) |
790 |
758 |
4.2 |
||||||||||||||||
|
Neuroscience |
1,394 |
1,397 |
(0.2) |
2,840 |
2,533 |
12.1 |
||||||||||||||||
|
Cranial & Spinal Technologies |
749 |
770 |
(2.7) |
1,544 |
1,462 |
5.6 |
||||||||||||||||
|
Specialty Therapies |
354 |
346 |
2.3 |
714 |
588 |
21.4 |
||||||||||||||||
|
Neuromodulation |
291 |
281 |
3.6 |
582 |
483 |
20.5 |
||||||||||||||||
|
Diabetes |
261 |
284 |
(8.1) |
506 |
572 |
(11.5) |
||||||||||||||||
|
TOTAL |
$ |
3,997 |
$ |
4,054 |
(1.4) |
% |
$ |
8,098 |
$ |
7,405 |
9.4 |
% |
||||||||||
|
(1) U.S. includes the United States and U.S. territories. |
|
(2) The data in this schedule has been intentionally rounded to the nearest million and, therefore, may not sum. |
|
(3) In the fourth quarter of fiscal year 2021, the Company realigned its divisions within Cardiovascular. As a result, fiscal year 2021 results have been recast to adjust for this realignment. |
|
MEDTRONIC PLC WORLD WIDE REVENUE: GEOGRAPHIC (1)(2) (Unaudited) |
||||||||||||||||||||||||||||||||||||||||||||
|
SECOND QUARTER |
SECOND QUARTER YEAR-TO-DATE(3) |
|||||||||||||||||||||||||||||||||||||||||||
|
REPORTED |
CONSTANT |
REPORTED |
CONSTANT |
|||||||||||||||||||||||||||||||||||||||||
|
(in millions) |
FY22 |
FY21 |
Growth |
Currency |
FY22 |
Growth |
FY22 |
FY21 |
Growth |
Currency |
FY22 |
Growth |
||||||||||||||||||||||||||||||||
|
U.S. |
$ |
1,373 |
$ |
1,377 |
(0.3) |
% |
$ |
— |
$ |
1,373 |
(0.3) |
% |
$ |
2,793 |
$ |
2,582 |
8.2 |
% |
$ |
— |
$ |
2,793 |
8.2 |
% |
||||||||||||||||||||
|
Non-U.S. Developed |
948 |
945 |
0.3 |
(2) |
950 |
0.5 |
1,952 |
1,798 |
8.6 |
69 |
1,883 |
4.7 |
||||||||||||||||||||||||||||||||
|
Emerging Markets |
506 |
404 |
25.2 |
13 |
493 |
22.0 |
972 |
778 |
24.9 |
37 |
935 |
20.2 |
||||||||||||||||||||||||||||||||
|
Cardiovascular |
2,827 |
2,725 |
3.7 |
11 |
2,816 |
3.3 |
5,717 |
5,158 |
10.8 |
106 |
5,611 |
8.8 |
||||||||||||||||||||||||||||||||
|
U.S. |
970 |
996 |
(2.6) |
— |
970 |
(2.6) |
1,959 |
1,718 |
14.0 |
— |
1,959 |
14.0 |
||||||||||||||||||||||||||||||||
|
Non-U.S. Developed |
841 |
837 |
0.5 |
(3) |
844 |
0.8 |
1,710 |
1,556 |
9.9 |
54 |
1,656 |
6.4 |
||||||||||||||||||||||||||||||||
|
Emerging Markets |
488 |
452 |
8.0 |
11 |
477 |
5.5 |
951 |
811 |
17.3 |
31 |
920 |
13.4 |
||||||||||||||||||||||||||||||||
|
Medical Surgical |
2,299 |
2,285 |
0.6 |
8 |
2,291 |
0.3 |
4,621 |
4,086 |
13.1 |
85 |
4,536 |
11.0 |
||||||||||||||||||||||||||||||||
|
U.S. |
1,394 |
1,397 |
(0.2) |
— |
1,394 |
(0.2) |
2,840 |
2,533 |
12.1 |
— |
2,840 |
12.1 |
||||||||||||||||||||||||||||||||
|
Non-U.S. Developed |
433 |
426 |
1.6 |
(1) |
434 |
1.9 |
898 |
802 |
12.0 |
28 |
870 |
8.5 |
||||||||||||||||||||||||||||||||
|
Emerging Markets |
309 |
240 |
28.8 |
11 |
298 |
24.2 |
602 |
439 |
37.1 |
29 |
573 |
30.5 |
||||||||||||||||||||||||||||||||
|
Neuroscience |
2,136 |
2,063 |
3.5 |
10 |
2,126 |
3.1 |
4,340 |
3,774 |
15.0 |
57 |
4,283 |
13.5 |
||||||||||||||||||||||||||||||||
|
U.S. |
261 |
284 |
(8.1) |
— |
261 |
(8.1) |
506 |
572 |
(11.5) |
— |
506 |
(11.5) |
||||||||||||||||||||||||||||||||
|
Non-U.S. Developed |
256 |
238 |
7.6 |
2 |
254 |
6.7 |
519 |
465 |
11.6 |
25 |
494 |
6.2 |
||||||||||||||||||||||||||||||||
|
Emerging Markets |
69 |
51 |
35.3 |
1 |
68 |
33.3 |
132 |
100 |
32.0 |
4 |
128 |
28.0 |
||||||||||||||||||||||||||||||||
|
Diabetes |
585 |
574 |
1.9 |
3 |
582 |
1.4 |
1,157 |
1,136 |
1.8 |
29 |
1,128 |
(0.7) |
||||||||||||||||||||||||||||||||
|
U.S. |
3,997 |
4,054 |
(1.4) |
— |
3,997 |
(1.4) |
8,098 |
7,405 |
9.4 |
— |
8,098 |
9.4 |
||||||||||||||||||||||||||||||||
|
Non-U.S. Developed |
2,478 |
2,446 |
1.3 |
(5) |
2,483 |
1.5 |
5,079 |
4,621 |
9.9 |
177 |
4,902 |
6.1 |
||||||||||||||||||||||||||||||||
|
Emerging Markets |
1,372 |
1,147 |
19.6 |
37 |
1,335 |
16.4 |
2,658 |
2,128 |
24.9 |
100 |
2,558 |
20.2 |
||||||||||||||||||||||||||||||||
|
TOTAL |
$ |
7,847 |
$ |
7,647 |
2.6 |
% |
$ |
32 |
$ |
7,815 |
2.2 |
% |
$ |
15,835 |
$ |
14,154 |
11.9 |
% |
$ |
277 |
$ |
15,558 |
9.9 |
% |
||||||||||||||||||||
|
(1) U.S. includes the United States and U.S. territories. Non-U.S. developed markets include Japan, Australia, New Zealand, Korea, Canada, and the countries of Western Europe. Emerging Markets include the countries of the Middle East, Africa, Latin America, Eastern Europe, and the countries of Asia that are not included in the non-U.S. developed markets, as previously defined. |
|
(2) The data in this schedule has been intentionally rounded to the nearest million and, therefore, may not sum. |
|
(3) Fiscal year 2021 was a 53-week fiscal year, with the extra week occurring in the first fiscal month of the first quarter and included in reported prior year second quarter year-to-date results. While it is difficult to calculate the impact of the extra week, the Company estimates the extra week benefited the prior year second quarter year-to-date revenue by approximately $360 to $390 million. |
|
(4) The currency impact to revenue measures the change in revenue between current and prior year periods using constant exchange rates. |
|
MEDTRONIC PLC CONSOLIDATED STATEMENTS OF INCOME (Unaudited) |
|||||||||||||||
|
Three months ended |
Six months ended |
||||||||||||||
|
(in millions, except per share data) |
October 29, 2021 |
October 30, 2020 |
October 29, 2021 |
October 30, 2020 |
|||||||||||
|
Net sales |
$ |
7,847 |
$ |
7,647 |
$ |
15,835 |
$ |
14,154 |
|||||||
|
Costs and expenses: |
|||||||||||||||
|
Cost of products sold |
2,497 |
2,705 |
5,095 |
5,209 |
|||||||||||
|
Research and development expense |
676 |
639 |
1,426 |
1,260 |
|||||||||||
|
Selling, general, and administrative expense |
2,615 |
2,600 |
5,163 |
5,017 |
|||||||||||
|
Amortization of intangible assets |
431 |
443 |
866 |
884 |
|||||||||||
|
Restructuring charges, net |
10 |
97 |
21 |
150 |
|||||||||||
|
Certain litigation charges, net |
34 |
84 |
60 |
(4) |
|||||||||||
|
Other operating expense, net |
21 |
149 |
781 |
35 |
|||||||||||
|
Operating profit |
1,563 |
930 |
2,422 |
1,603 |
|||||||||||
|
Other non-operating income, net |
(66) |
(65) |
(177) |
(147) |
|||||||||||
|
Interest expense |
136 |
470 |
273 |
641 |
|||||||||||
|
Income before income taxes |
1,493 |
525 |
2,326 |
1,109 |
|||||||||||
|
Income tax provision |
176 |
31 |
240 |
124 |
|||||||||||
|
Net income |
1,317 |
494 |
2,086 |
985 |
|||||||||||
|
Net income attributable to noncontrolling interests |
(6) |
(5) |
(12) |
(9) |
|||||||||||
|
Net income attributable to Medtronic |
$ |
1,311 |
$ |
489 |
$ |
2,074 |
$ |
976 |
|||||||
|
Basic earnings per share |
$ |
0.97 |
$ |
0.36 |
$ |
1.54 |
$ |
0.73 |
|||||||
|
Diluted earnings per share |
$ |
0.97 |
$ |
0.36 |
$ |
1.53 |
$ |
0.72 |
|||||||
|
Basic weighted average shares outstanding |
1,345.1 |
1,344.4 |
1,344.8 |
1,343.1 |
|||||||||||
|
Diluted weighted average shares outstanding |
1,355.3 |
1,352.1 |
1,355.9 |
1,351.1 |
|||||||||||
|
The data in this schedule has been intentionally rounded to the nearest million, and, therefore, may not sum. |
|
MEDTRONIC PLC GAAP TO NON-GAAP RECONCILIATIONS(1) (Unaudited) |
||||||||||||||||||||||||||||||||
|
Three months ended October 29, 2021 |
||||||||||||||||||||||||||||||||
|
(in millions, except per share data) |
Net |
Cost of |
Gross |
Operating |
Operating |
Income |
Net Income |
Diluted |
Effective |
|||||||||||||||||||||||
|
GAAP |
$ |
7,847 |
$ |
2,497 |
68.2 |
% |
$ |
1,563 |
19.9 |
% |
$ |
1,493 |
$ |
1,311 |
$ |
0.97 |
11.8 |
% |
||||||||||||||
|
Non-GAAP Adjustments: |
||||||||||||||||||||||||||||||||
|
Restructuring and associated costs (2) |
— |
(31) |
0.4 |
77 |
1.0 |
77 |
62 |
0.05 |
19.5 |
|||||||||||||||||||||||
|
Acquisition-related items (3) |
— |
(5) |
0.1 |
(13) |
(0.2) |
(13) |
(15) |
(0.01) |
(15.4) |
|||||||||||||||||||||||
|
Certain litigation charges |
— |
— |
— |
34 |
0.4 |
34 |
30 |
0.02 |
11.8 |
|||||||||||||||||||||||
|
(Gain)/loss on minority investments (4) |
— |
— |
— |
— |
— |
6 |
6 |
— |
— |
|||||||||||||||||||||||
|
Medical device regulations (5) |
— |
(15) |
0.2 |
24 |
0.3 |
24 |
20 |
0.01 |
16.7 |
|||||||||||||||||||||||
|
Amortization of intangible assets |
— |
— |
— |
431 |
5.5 |
431 |
361 |
0.27 |
16.0 |
|||||||||||||||||||||||
|
Certain tax adjustments, net (6) |
— |
— |
— |
— |
— |
— |
16 |
0.01 |
— |
|||||||||||||||||||||||
|
Non-GAAP |
$ |
7,847 |
$ |
2,447 |
68.8 |
% |
$ |
2,116 |
27.0 |
% |
$ |
2,052 |
$ |
1,792 |
$ |
1.32 |
12.4 |
% |
||||||||||||||
|
Currency impact |
(32) |
30 |
(0.5) |
(58) |
(0.7) |
(0.04) |
||||||||||||||||||||||||||
|
Currency Adjusted |
$ |
7,815 |
$ |
2,477 |
68.3 |
% |
$ |
2,058 |
26.3 |
% |
$ |
1.28 |
||||||||||||||||||||
|
Three months ended October 30, 2020 |
||||||||||||||||||||||||||||||||
|
(in millions, except per share data) |
Net |
Cost of |
Gross |
Operating |
Operating |
Income |
Net Income |
Diluted |
Effective |
|||||||||||||||||||||||
|
GAAP |
$ |
7,647 |
$ |
2,705 |
64.6 |
% |
$ |
930 |
12.2 |
% |
$ |
525 |
$ |
489 |
$ |
0.36 |
5.9 |
% |
||||||||||||||
|
Non-GAAP Adjustments: |
||||||||||||||||||||||||||||||||
|
Restructuring and associated costs (2) |
— |
(32) |
0.4 |
179 |
2.3 |
179 |
135 |
0.10 |
24.6 |
|||||||||||||||||||||||
|
Acquisition-related items (3) |
— |
(2) |
— |
47 |
0.6 |
47 |
39 |
0.03 |
17.0 |
|||||||||||||||||||||||
|
Certain litigation charges |
— |
— |
— |
84 |
1.1 |
84 |
63 |
0.05 |
25.0 |
|||||||||||||||||||||||
|
(Gain)/loss on minority investments (4) |
— |
— |
— |
— |
— |
1 |
1 |
— |
— |
|||||||||||||||||||||||
|
Medical device regulations (5) |
— |
(11) |
0.1 |
19 |
0.2 |
19 |
16 |
0.01 |
15.8 |
|||||||||||||||||||||||
|
Amortization of intangible assets |
— |
— |
— |
443 |
5.8 |
443 |
373 |
0.28 |
15.8 |
|||||||||||||||||||||||
|
Debt tender premium and other charges (7) |
— |
— |
— |
— |
— |
308 |
248 |
0.18 |
19.5 |
|||||||||||||||||||||||
|
Certain tax adjustments, net (6) |
— |
— |
— |
— |
— |
— |
16 |
0.01 |
— |
|||||||||||||||||||||||
|
Non-GAAP |
$ |
7,647 |
$ |
2,660 |
65.2 |
% |
$ |
1,702 |
22.3 |
% |
$ |
1,606 |
$ |
1,380 |
$ |
1.02 |
13.8 |
% |
||||||||||||||
|
See description of non-GAAP financial measures at the end of the earnings press release. |
|
(1) The data in this schedule has been intentionally rounded to the nearest million or $0.01 for EPS figures, and, therefore, may not sum. |
|
(2) Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses. |
|
(3) The charges primarily include business combination costs, changes in fair value of contingent consideration, and for the three months ended October 30, 2020, certain license payments for unapproved technology. |
|
(4) We exclude unrealized and realized gains and losses on our minority investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations. |
|
(5) The charges represent incremental costs of complying with the new European Union (E.U.) medical device regulations for previously registered products and primarily include charges for contractors supporting the project and other direct third-party expenses. |
|
(6) The charges include the amortization on previously established deferred tax assets from intercompany intellectual property transactions. |
|
(7) The charges relate to the early redemption of approximately $6.0 billion of debt. |
|
MEDTRONIC PLC GAAP TO NON-GAAP RECONCILIATIONS(1) (Unaudited) |
||||||||||||||||||||||||||||||||
|
Six months ended October 29, 2021 |
||||||||||||||||||||||||||||||||
|
(in millions, except per share data) |
Net |
Cost of |
Gross |
Operating |
Operating |
Income |
Net Income |
Diluted |
Effective |
|||||||||||||||||||||||
|
GAAP |
$ |
15,835 |
$ |
5,095 |
67.8 |
% |
$ |
2,422 |
15.3 |
% |
$ |
2,326 |
$ |
2,074 |
$ |
1.53 |
10.3 |
% |
||||||||||||||
|
Non-GAAP Adjustments: |
||||||||||||||||||||||||||||||||
|
Restructuring and associated costs (2) |
— |
(64) |
0.4 |
159 |
1.0 |
159 |
128 |
0.09 |
19.5 |
|||||||||||||||||||||||
|
Acquisition-related items (3) |
— |
(9) |
0.1 |
96 |
0.6 |
96 |
72 |
0.05 |
25.0 |
|||||||||||||||||||||||
|
Certain litigation charges |
— |
— |
— |
60 |
0.4 |
60 |
51 |
0.04 |
15.0 |
|||||||||||||||||||||||
|
(Gain)/loss on minority investments (4) |
— |
— |
— |
— |
— |
(25) |
(22) |
(0.02) |
— |
|||||||||||||||||||||||
|
Medical device regulations (5) |
— |
(26) |
0.2 |
45 |
0.3 |
45 |
36 |
0.03 |
20.0 |
|||||||||||||||||||||||
|
Amortization of intangible assets |
— |
— |
— |
866 |
5.5 |
866 |
728 |
0.54 |
16.1 |
|||||||||||||||||||||||
|
MCS impairments / costs (6) |
— |
(58) |
0.4 |
726 |
4.6 |
726 |
564 |
0.42 |
22.3 |
|||||||||||||||||||||||
|
Certain tax adjustments, net (7) |
— |
— |
— |
— |
— |
— |
69 |
0.05 |
— |
|||||||||||||||||||||||
|
Non-GAAP |
$ |
15,835 |
$ |
4,938 |
68.8 |
% |
$ |
4,374 |
27.6 |
% |
$ |
4,253 |
$ |
3,699 |
$ |
2.73 |
12.8 |
% |
||||||||||||||
|
Currency impact |
(277) |
(26) |
(0.4) |
(105) |
(0.2) |
(0.07) |
||||||||||||||||||||||||||
|
Currency Adjusted |
$ |
15,558 |
$ |
4,912 |
68.4 |
% |
$ |
4,269 |
27.4 |
% |
$ |
2.66 |
||||||||||||||||||||
|
Six months ended October 30, 2020 |
||||||||||||||||||||||||||||||||
|
(in millions, except per share data) |
Net |
Cost of |
Gross |
Operating |
Operating |
Income |
Net Income |
Diluted |
Effective |
|||||||||||||||||||||||
|
GAAP |
$ |
14,154 |
$ |
5,209 |
63.2 |
% |
$ |
1,603 |
11.3 |
% |
$ |
1,109 |
$ |
976 |
$ |
0.72 |
11.2 |
% |
||||||||||||||
|
Non-GAAP Adjustments: |
||||||||||||||||||||||||||||||||
|
Restructuring and associated costs (2) |
— |
(59) |
0.4 |
307 |
2.2 |
307 |
241 |
0.18 |
21.5 |
|||||||||||||||||||||||
|
Acquisition-related items (3) |
— |
(5) |
— |
(49) |
(0.3) |
(49) |
(28) |
(0.02) |
42.9 |
|||||||||||||||||||||||
|
Certain litigation charges |
— |
— |
— |
(4) |
— |
(4) |
(6) |
— |
(50.0) |
|||||||||||||||||||||||
|
(Gain)/loss on minority investments (4) |
— |
— |
— |
— |
— |
(9) |
(10) |
(0.01) |
(11.1) |
|||||||||||||||||||||||
|
Medical device regulations (5) |
— |
(20) |
0.1 |
37 |
0.3 |
37 |
32 |
0.02 |
13.5 |
|||||||||||||||||||||||
|
Amortization of intangible assets |
— |
— |
— |
884 |
6.2 |
884 |
743 |
0.55 |
16.0 |
|||||||||||||||||||||||
|
Debt tender premium and other charges (8) |
— |
— |
— |
— |
— |
308 |
248 |
0.18 |
19.5 |
|||||||||||||||||||||||
|
Certain tax adjustments, net (7) |
— |
— |
— |
— |
— |
— |
20 |
0.01 |
— |
|||||||||||||||||||||||
|
Non-GAAP |
$ |
14,154 |
$ |
5,125 |
63.8 |
% |
$ |
2,778 |
19.6 |
% |
$ |
2,583 |
$ |
2,216 |
$ |
1.64 |
13.9 |
% |
||||||||||||||
|
See description of non-GAAP financial measures contained in this release. |
|
(1) The data in this schedule has been intentionally rounded to the nearest million or $0.01 for EPS figures, and, therefore, may not sum. |
|
(2) Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses. |
|
(3) The charges primarily include business combination costs, changes in fair value of contingent consideration, and specifically for the six months ended October 30, 2020, change in amounts accrued for certain contingent liabilities for recent acquisitions. |
|
(4) We exclude unrealized and realized gains and losses on our minority investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations. |
|
(5) The charges represent incremental costs of complying with the new E.U. medical device regulations for previously registered products and primarily include charges for contractors supporting the project and other direct third-party expenses. |
|
(6) The charges relate to the Company's June 2021 decision to stop the distribution and sale of the Medtronic HVAD System within the Mechanical Circulatory Support Operating Unit (MCS). The charges included $515 million of non-cash impairments, primarily related to $409 million of intangible asset impairments, as well as $211 million for commitments and obligations in connection with the decision, including customer support obligations, restructuring, and other associated costs. Medtronic is committed to serving the needs of the approximately 4,000 patients currently implanted with the HVAD System. |
|
(7) The charges include the amortization on previously established deferred tax assets from intercompany intellectual property transactions, and specifically for the six months ended October 29, 2021, charges associated with a change in the company's permanent reinvestment assertion on certain historical earnings. |
|
(8) The charges relate to the early redemption of approximately $6.0 billion of debt. |
|
MEDTRONIC PLC GAAP TO NON-GAAP RECONCILIATIONS(1) (Unaudited) |
||||||||||||||||||||||||||||
|
Three months ended October 29, 2021 |
||||||||||||||||||||||||||||
|
(in millions) |
Net |
SG&A |
SG&A |
R&D |
R&D |
Other |
Other |
Other Non- |
||||||||||||||||||||
|
GAAP |
$ |
7,847 |
$ |
2,615 |
33.3 |
% |
$ |
676 |
8.6 |
% |
$ |
21 |
0.3 |
% |
$ |
(66) |
||||||||||||
|
Non-GAAP Adjustments: |
||||||||||||||||||||||||||||
|
Restructuring and associated costs (2) |
— |
(37) |
(0.5) |
— |
— |
— |
— |
— |
||||||||||||||||||||
|
Acquisition-related items (3) |
— |
— |
— |
— |
— |
17 |
0.2 |
— |
||||||||||||||||||||
|
Medical device regulations (4) |
— |
— |
— |
(9) |
(0.1) |
— |
— |
— |
||||||||||||||||||||
|
Gain/(loss) on minority investments (5) |
— |
— |
— |
— |
— |
— |
— |
(6) |
||||||||||||||||||||
|
Non-GAAP |
$ |
7,847 |
$ |
2,578 |
32.9 |
% |
$ |
667 |
8.5 |
% |
$ |
39 |
0.5 |
% |
$ |
(72) |
||||||||||||
|
Currency impact |
(32) |
(11) |
(0.1) |
(2) |
— |
9 |
0.1 |
— |
||||||||||||||||||||
|
Currency Adjusted |
$ |
7,815 |
$ |
2,567 |
32.8 |
% |
$ |
665 |
8.5 |
% |
$ |
48 |
0.6 |
% |
$ |
(72) |
||||||||||||
|
Six months ended October 29, 2021 |
||||||||||||||||||||||||||||
|
(in millions) |
Net |
SG&A |
SG&A |
R&D |
R&D |
Other |
Other |
Other Non- |
||||||||||||||||||||
|
GAAP |
$ |
15,835 |
$ |
5,163 |
32.6 |
% |
$ |
1,426 |
9.0 |
% |
$ |
781 |
4.9 |
% |
$ |
(177) |
||||||||||||
|
Non-GAAP Adjustments: |
||||||||||||||||||||||||||||
|
Restructuring and associated costs (2) |
— |
(74) |
(0.5) |
— |
— |
— |
— |
— |
||||||||||||||||||||
|
Acquisition-related items (3) |
— |
— |
— |
(90) |
(0.6) |
4 |
— |
— |
||||||||||||||||||||
|
Medical device regulations (4) |
— |
(1) |
— |
(18) |
(0.1) |
— |
— |
— |
||||||||||||||||||||
|
MCS impairment / costs (6) |
— |
— |
— |
— |
— |
(668) |
(4.2) |
— |
||||||||||||||||||||
|
Gain/(loss) on minority investments (5) |
— |
— |
— |
— |
— |
— |
— |
25 |
||||||||||||||||||||
|
Non-GAAP |
$ |
15,835 |
$ |
5,087 |
32.1 |
% |
$ |
1,318 |
8.3 |
% |
$ |
118 |
0.7 |
% |
$ |
(152) |
||||||||||||
|
Currency impact |
(277) |
(80) |
0.1 |
(9) |
0.1 |
(57) |
(0.3) |
1 |
||||||||||||||||||||
|
Currency Adjusted |
$ |
15,558 |
$ |
5,007 |
32.2 |
% |
$ |
1,309 |
8.4 |
% |
$ |
61 |
0.4 |
% |
$ |
(151) |
||||||||||||
|
See description of non-GAAP financial measures at the end of the earnings press release. |
|
(1) The data in this schedule has been intentionally rounded to the nearest million, and, therefore, may not sum. |
|
(2) Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses. |
|
(3) The charges primarily includeusiness combination costs, changes in fair value of contingent consideration, and specifically in the six months ended October 29, 2021, acquisitions of, and certain license payments for, unapproved technology. |
|
(4) The charges represent incremental costs of complying with the new E.U. medical device regulations for previously registered products and primarily include charges for contractors supporting the project and other direct third-party expenses. |
|
(5) We exclude unrealized and realized gains and losses on our minority investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations. |
|
(6) The charges relate to the Company's June 2021 decision to stop the distribution and sale of the Medtronic HVAD System within the Mechanical Circulatory Support Operating Unit (MCS). The charges included $515 million of non-cash impairments, primarily related to $409 million of intangible asset impairments, as well as $211 million for commitments and obligations in connection with the decision, including customer support obligations, restructuring, and other associated costs. Medtronic is committed to serving the needs of the approximately 4,000 patients currently implanted with the HVAD System. |
|
MEDTRONIC PLC GAAP TO NON-GAAP RECONCILIATIONS(1) (Unaudited) |
|||||||||||
|
Six months |
Six months |
Fiscal year |
|||||||||
|
(in millions) |
October 29, 2021 |
October 30, 2020 |
2021 |
||||||||
|
Net cash provided by operating activities |
$ |
3,061 |
$ |
2,139 |
$ |
6,240 |
|||||
|
Additions to property, plant, and equipment |
(649) |
(615) |
(1,355) |
||||||||
|
Free Cash Flow (2) |
$ |
2,412 |
$ |
1,524 |
$ |
4,885 |
|||||
|
See description of non-GAAP financial measures at the end of the earnings press release. |
|
(1) The data in this schedule has been intentionally rounded to the nearest million, and, therefore, may not sum. |
|
(2) Free cash flow represents operating cash flows less property, plant, and equipment additions. |
|
MEDTRONIC PLC CONSOLIDATED BALANCE SHEETS (Unaudited) |
||||||||
|
(in millions) |
October 29, 2021 |
April 30, 2021 |
||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ |
2,900 |
$ |
3,593 |
||||
|
Investments |
7,769 |
7,224 |
||||||
|
Accounts receivable, less allowances and credit losses of $255 and $241, respectively |
5,493 |
5,462 |
||||||
|
Inventories, net |
4,349 |
4,313 |
||||||
|
Other current assets |
2,220 |
1,955 |
||||||
|
Total current assets |
22,731 |
22,548 |
||||||
|
Property, plant, and equipment |
12,978 |
12,700 |
||||||
|
Accumulated depreciation |
(7,790) |
(7,479) |
||||||
|
Property, plant, and equipment, net |
5,188 |
5,221 |
||||||
|
Goodwill |
41,612 |
41,961 |
||||||
|
Other intangible assets, net |
16,523 |
17,740 |
||||||
|
Tax assets |
3,203 |
3,169 |
||||||
|
Other assets |
2,499 |
2,443 |
||||||
|
Total assets |
$ |
91,756 |
$ |
93,083 |
||||
|
LIABILITIES AND EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Current debt obligations |
$ |
16 |
$ |
11 |
||||
|
Accounts payable |
1,917 |
2,106 |
||||||
|
Accrued compensation |
1,934 |
2,482 |
||||||
|
Accrued income taxes |
467 |
435 |
||||||
|
Other accrued expenses |
3,469 |
3,475 |
||||||
|
Total current liabilities |
7,803 |
8,509 |
||||||
|
Long-term debt |
25,607 |
26,378 |
||||||
|
Accrued compensation and retirement benefits |
1,505 |
1,557 |
||||||
|
Accrued income taxes |
2,110 |
2,251 |
||||||
|
Deferred tax liabilities |
1,024 |
1,028 |
||||||
|
Other liabilities |
1,547 |
1,756 |
||||||
|
Total liabilities |
39,596 |
41,481 |
||||||
|
Commitments and contingencies |
||||||||
|
Shareholders' equity: |
||||||||
|
Ordinary shares— par value $0.0001, 2.6 billion shares authorized, 1,344,861,769 and |
— |
— |
||||||
|
Additional paid-in capital |
26,059 |
26,319 |
||||||
|
Retained earnings |
28,974 |
28,594 |
||||||
|
Accumulated other comprehensive loss |
(3,042) |
(3,485) |
||||||
|
Total shareholders' equity |
51,991 |
51,428 |
||||||
|
Noncontrolling interests |
168 |
174 |
||||||
|
Total equity |
52,159 |
51,602 |
||||||
|
Total liabilities and equity |
$ |
91,756 |
$ |
93,083 |
||||
|
The data in this schedule has been intentionally rounded to the nearest million, and, therefore, may not sum. |
|
MEDTRONIC PLC CONSOLIDATED STATEMENTS OF CASH FLOWS (Unaudited) |
||||||||
|
Six months ended |
||||||||
|
(in millions) |
October 29, 2021 |
October 30, 2020 |
||||||
|
Operating Activities: |
||||||||
|
Net income |
$ |
2,086 |
$ |
985 |
||||
|
Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||
|
Depreciation and amortization |
1,347 |
1,340 |
||||||
|
Provision for doubtful accounts |
34 |
86 |
||||||
|
Deferred income taxes |
(78) |
(69) |
||||||
|
Stock-based compensation |
209 |
210 |
||||||
|
Loss on debt extinguishment |
— |
308 |
||||||
|
MCS asset impairment and inventory write-down |
515 |
— |
||||||
|
Other, net |
130 |
112 |
||||||
|
Change in operating assets and liabilities, net of acquisitions and divestitures: |
||||||||
|
Accounts receivable, net |
(171) |
(669) |
||||||
|
Inventories |
(156) |
(145) |
||||||
|
Accounts payable and accrued liabilities |
(446) |
108 |
||||||
|
Other operating assets and liabilities |
(409) |
(127) |
||||||
|
Net cash provided by operating activities |
3,061 |
2,139 |
||||||
|
Investing Activities: |
||||||||
|
Acquisitions, net of cash acquired |
(91) |
(370) |
||||||
|
Additions to property, plant, and equipment |
(649) |
(615) |
||||||
|
Purchases of investments |
(5,311) |
(5,360) |
||||||
|
Sales and maturities of investments |
4,637 |
4,337 |
||||||
|
Other investing activities, net |
(79) |
(4) |
||||||
|
Net cash used in investing activities |
(1,493) |
(2,012) |
||||||
|
Financing Activities: |
||||||||
|
Change in current debt obligations, net |
— |
(57) |
||||||
|
Proceeds from short-term borrowings (maturities greater than 90 days) |
— |
2,789 |
||||||
|
Issuance of long-term debt |
— |
7,172 |
||||||
|
Payments on long-term debt |
(1) |
(6,336) |
||||||
|
Dividends to shareholders |
(1,693) |
(1,558) |
||||||
|
Issuance of ordinary shares |
274 |
119 |
||||||
|
Repurchase of ordinary shares |
(744) |
(68) |
||||||
|
Other financing activities |
(46) |
(70) |
||||||
|
Net cash provided by (used in) financing activities |
(2,210) |
1,991 |
||||||
|
Effect of exchange rate changes on cash and cash equivalents |
(51) |
162 |
||||||
|
Net change in cash and cash equivalents |
(693) |
2,280 |
||||||
|
Cash and cash equivalents at beginning of period |
3,593 |
4,140 |
||||||
|
Cash and cash equivalents at end of period |
$ |
2,900 |
$ |
6,420 |
||||
|
Supplemental Cash Flow Information |
||||||||
|
Cash paid for: |
||||||||
|
Income taxes |
$ |
615 |
$ |
384 |
||||
|
Interest |
280 |
321 |
||||||
|
The data in this schedule has been intentionally rounded to the nearest million, and, therefore, may not sum. |
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
FORWARD LOOKING STATEMENTS
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are subject to risks and uncertainties, including risks related to competitive factors, difficulties and delays inherent in the development, manufacturing, marketing and sale of medical products, government regulation and general economic conditions and other risks and uncertainties described in the company's periodic reports on file with the U.S. Securities and Exchange Commission including the most recent Annual Report on Form 10-K of the company, as filed with the U.S. Securities and Exchange Commission. In some cases, you can identify these statements by forward-looking words or expressions, such as "anticipate," "believe," "could," "estimate," "expect," "forecast," "intend," "looking ahead," "may," "plan," "possible," "potential," "project," "should," "going to," "will," and similar words or expressions, the negative or plural of such words or expressions and other comparable terminology. Actual results may differ materially from anticipated results. Medtronic does not undertake to update its forward-looking statements or any of the information contained in this press release, including to reflect future events or circumstances.
NON-GAAP FINANCIAL MEASURES
This press release contains financial measures, including adjusted net income, adjusted diluted EPS, and organic revenue, which are considered "non-GAAP" financial measures under applicable SEC rules and regulations. References to quarterly figures increasing, decreasing or remaining flat are in comparison to the second quarter of fiscal year 2021.
Medtronic management believes that non-GAAP financial measures provide information useful to investors in understanding the company's underlying operational performance and trends and to facilitate comparisons with the performance of other companies in the med tech industry. Non-GAAP net income and diluted EPS exclude the effect of certain charges or gains that contribute to or reduce earnings but that result from transactions or events that management believes may or may not recur with similar materiality or impact to operations in future periods (Non-GAAP Adjustments). Medtronic generally uses non-GAAP financial measures to facilitate management's review of the operational performance of the company and as a basis for strategic planning. Non-GAAP financial measures should be considered supplemental to and not a substitute for financial information prepared in accordance with U.S. generally accepted accounting principles (GAAP), and investors are cautioned that Medtronic may calculate non-GAAP financial measures in a way that is different from other companies. Management strongly encourages investors to review the company's consolidated financial statements and publicly filed reports in their entirety. Reconciliations of the non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the financial schedules accompanying this press release.
Medtronic calculates forward-looking non-GAAP financial measures based on internal forecasts that omit certain amounts that would be included in GAAP financial measures. For instance, forward-looking organic revenue growth guidance excludes the impact of foreign currency fluctuations, as well as significant acquisitions or divestitures. Forward-looking diluted non-GAAP EPS guidance also excludes other potential charges or gains that would be recorded as Non-GAAP Adjustments to earnings during the fiscal year. Medtronic does not attempt to provide reconciliations of forward-looking non-GAAP EPS guidance to projected GAAP EPS guidance because the combined impact and timing of recognition of these potential charges or gains is inherently uncertain and difficult to predict and is unavailable without unreasonable efforts. In addition, the company believes such reconciliations would imply a degree of precision and certainty that could be confusing to investors. Such items could have a substantial impact on GAAP measures of financial performance.
|
Contacts: |
|
|
Erika Winkels |
Ryan Weispfenning |
|
Public Relations |
Investor Relations |
|
+1-763-526-8478 |
+1-763-505-4626 |
SOURCE Medtronic plc