Medtronic announces contract with Vizient for Touch Surgery™ Enterprise
The first AI-powered video management and analytics platform for the OR, Touch Surgery Enterprise gives surgical teams easy access to procedural videos and insights DUBLIN, March 9, 2022...
The first AI-powered video management and analytics platform for the OR, Touch Surgery Enterprise gives surgical teams easy access to procedural videos and insights
DUBLIN, March 9, 2022 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced it has entered into a contract with Vizient, a leading healthcare performance improvement company in the U.S., to add Touch Surgery™ Enterprise to Vizient's offerings, which serve more than half the nation's healthcare providers.
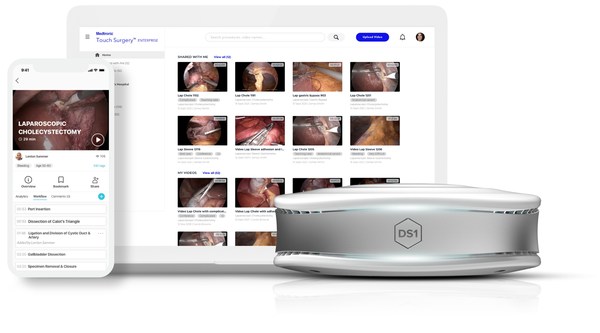
The first AI-powered surgical video management and analytics platform for the operating room (OR), Touch Surgery Enterprise significantly simplifies the process of recording, analyzing, and sharing surgical video — providing surgeons with a powerful new tool to improve performance and train others.1,†
A fully integrated hardware and software system connected to the cloud, Touch Surgery Enterprise works easily with many laparoscopic and robotic scopes, enabling hospitals to take the first step to digitizing their OR while leveraging existing equipment.
"Touch Surgery Enterprise allows surgeons to use data to refine the way surgery is taught and executed, and we believe that will raise the standard of healthcare for all," said George Murgatroyd, vice president and general manager, Digital Surgery within the Surgical Robotics business, which is part of the Medical Surgical Portfolio of Medtronic. "This agreement with Vizient allows us to build on the strong interest we're seeing globally, to support hospitals in the U.S. who are looking to harness the power of surgical video data and analysis."
Touch Surgery Enterprise is part of Medtronic's growing portfolio of artificial intelligence (AI) and surgical robotic solutions. It is compatible with the Hugo™ robotic-assisted surgery (RAS) system.‡
Vizient contract provides access to a nationwide network of hospitals
Vizient serves more than half the nation's acute care providers, including academic medical centers, community hospitals, pediatric facilities, and non-acute care providers.
"Vizient's mission is to help our member providers deliver high value care to the patients they serve. Our ability to evolve relationships with our suppliers is a critical piece to the equation as we look to deliver on a more comprehensive value proposition in the market," said Bryan Grossman, senior vice president, Strategic Supplier Performance & Category Management, Vizient. "Touch Surgery Enterprise aligns with our mission, offering our members the ability to analyze surgical processes that can help advance patient care."
AI-powered surgical video and data available anytime, anywhere
Touch Surgery Enterprise is comprised of the DS1 computer and controller — surgical video recording hardware designed specifically for the OR. Applications of built-in AI include:
- Automatic blurring of faces and protected information to ensure data privacy compliance before uploading surgical video to the AWS global cloud
- Automatic segmentation of surgical video into key procedural steps for a growing library of procedures
- Ability to benchmark a case against a bank of historical cases or make comparisons across departments
All videos are accessed through the Touch Surgery™ App, which is free to download and home to academically validated and accredited simulations for mobile training on 200+ procedures in 17+ specialties. With 2.5 million active users, the app allows surgeons to prepare, practice, and teach surgical procedures — anytime, anywhere.
"By simplifying the process to capture and analyze surgical video, Touch Surgery Enterprise gives surgical teams a powerful new tool to advance patient care," said Megan Rosengarten, president of the Surgical Robotics business at Medtronic. "We're excited about the impact it can make for customers today, as a solution for laparoscopic and robotic-assisted cases within their existing infrastructure, and for the possibility this technology creates in the future."
†Touch Surgery Enterprise is not intended to analyze medical images or signals to guide surgery, or aid in diagnosis or treatment of a disease or condition. It is available in most markets around the world, including in the U.S.
‡The Hugo RAS system is commercially available in certain geographies. Regulatory requirements of individual countries and regions will determine approval, clearance, or market availability. In the EU, the Hugo RAS system is CE marked. In Canada, the Hugo RAS system has a medical device license. The Hugo RAS system is approved in Australia. In the U.S., the Hugo RAS system is an investigational device not for sale.
For more information about Touch Surgery Enterprise, visit our website.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1. Based on a randomized controlled trial using porcine models: Singh P, Aggarwal R, Tahir M, Pucher PH, Darzi A. A randomized controlled study to evaluate the role of video-based coaching in training laparoscopic skills. Ann Surg. 2015;261(5):862–869.
Contacts: | |
Gary Jeanfaivre | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-203-833-2104 | +1-763-505-4626 |
SOURCE Medtronic plc