670G: The Math Guys who Made It Possible
Inside the MiniMed™ 670G system lies a math equation that took nearly a decade for Medtronic scientists to put together.
Doubts. Disagreements. And sleepless nights. It’s just a glimpse into the journey of discovering a math problem so complex, it’s changing the way diabetes is managed.
“You have to stick to what you believe in,” says Dr. Benyamin Grosman. “You have to stay focused on the patient impact.”
In 2008, Grosman, a Medtronic scientist who specializes in mathematics and control theory teamed up with Anirban Roy, a fellow Medtronic scientist with the same background. Their goal was to develop a sophisticated algorithm to assist a person managing their blood sugar levels.
“Math is what drives us - that’s our bread and butter,” says Roy.
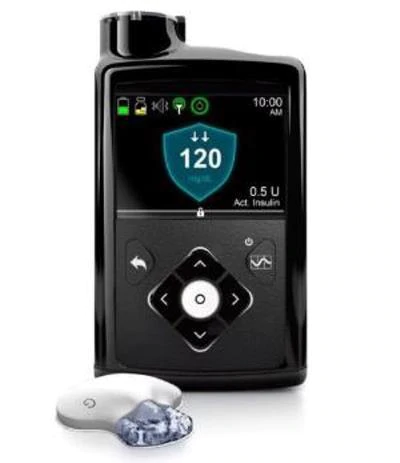
The algorithm would eventually be programmed inside the Medtronic MiniMed 670G system – the world’s first hybrid close looped insulin delivery system. It took nearly 10 years to put together, because the formula needed to mimic what the human body does naturally.
But that’s no easy math problem.
Blood sugar is constantly changing and from person to person, everyone is different. The algorithm, which would control the flow of insulin, needed to adjust to individual needs, how they responded to physical activity, how they absorb food, and so on.
They got to work.
“We’re talking about years, not days,” says Roy. “You do trials, you gain confidence, make tweaks, and return to a clinical environment. You have to have patience in healthcare, because you have to get it right.”
They were consumed. It meant middle-of-the-night emails and months of real-world testing. And they felt the pressure.
You have to have patience in healthcare, because you have to get it right.
Anirban Roy Medtronic scientist
“This is not just solving something in front of your laptop. This is impacting real people,” says Grosman. “But you to stick to what you believe in.”
When their obsession with a math problem was complete it became part of a first-of-its-kind system. The MiniMed 670G system features a highly-advanced sensor, the latest in insulin pump technology, and a dynamic algorithm working together to allow people living with diabetes an opportunity to worry less about their disease.
“This algorithm is learning the patient every day,” says Roy. “It’s keeping up with insulin sensitivity and has layers of safety built in.”
In the fall of 2016, the FDA gave the MiniMed 670G full approval. Today, the system is available to patients.
“There’s a ton of passion behind this project,” says Ali Dianaty, Vice President, R&D at Medtronic. “In diabetes, this is one of the biggest innovations of the last 10 years.”
And it’s changing people’s lives. Like Tia Geri, a 16-year old from California.
“I’ve heard from people who say it’s like taking a vacation from their disease,” says Roy. “That’s very fulfilling to hear.”
Roy and Grossman aren’t finished. They call the MiniMed 670G system “version one” and the work into a fully-automated system is already underway.
“We must keep innovating,” says Roy. “This is what we do.”
It’s the attitude that aligns with what Medtronic is all about – to alleviate pain, restore health, and extend life.
“People are moving into a new era – and we are a part of it,” says Grosman. “To me, this means a lot.”
Important Safety Information
The Medtronic MiniMed 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, fourteen years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed 670G system includes SmartGuard technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitor sensor glucose values, and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site (palm) or from a control solution test. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
Warning
Medtronic performed an evaluation of the MiniMed 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Only use rapid acting U100 insulin with this system. Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed 670G system has not been studied in pregnant women.
For complete details, including product and important safety information concerning the system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information#minimed-670g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library