Sep 17, 2020
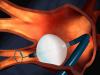
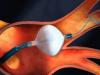
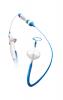
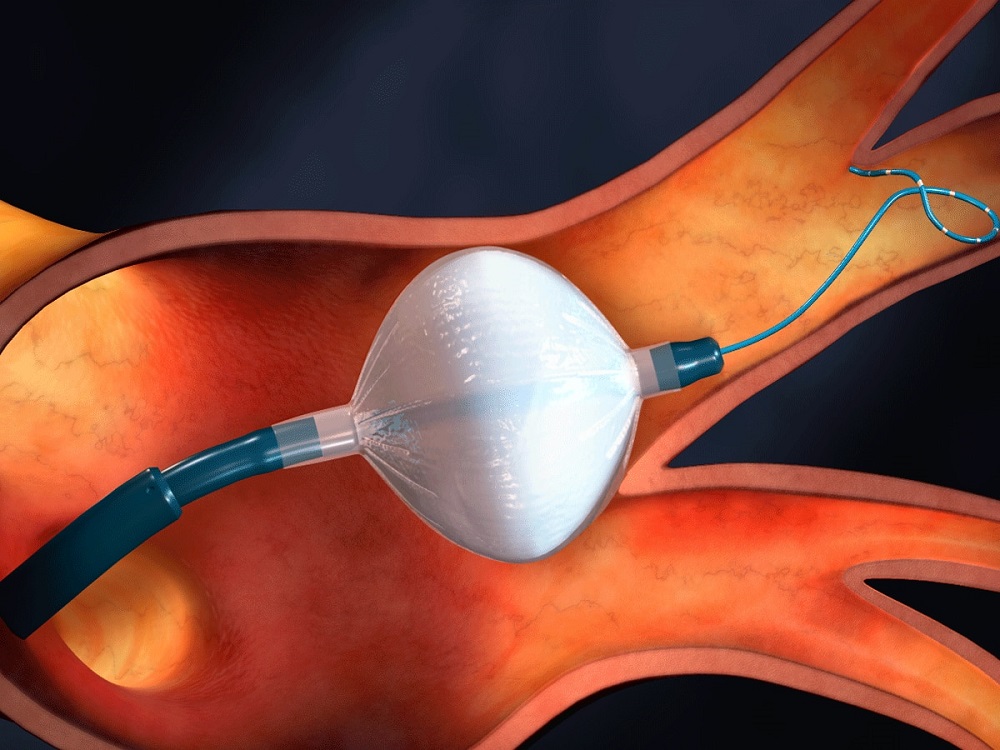
Arctic Front Advance™ Cardiac Cryoablation System
Information about the minimally invasive procedure to treat medication-resistant paroxysmal atrial fibrillation (PAF) using the Arctic Front Advance™ Cardiac Cryoablation System.
The following media kits are made available to provide reporters with background information on the range of Medtronic therapies and the conditions they treat.
Aug 1, 2017 Data from Landmark Trial Published in the Journal of the American Heart Association
|
|
Oct 13, 2016 Late Breaking Clinical Trial Demonstrates Favorable Health Economics and Positive Clinical Outcomes for AF Patients
|
|
Jun 10, 2016 Secondary Analyses Presented at Cardiostim 2016 Highlight Positive, Real-World Patient Outcomes
|
|
Apr 4, 2016 Cryoballoon Comparable in Safety and Effectiveness as Current Standard of Care Ablation Technology
|
|
May 12, 2015 Third-Generation Cryoballoon Designed to Allow Enhanced Positioning and Help Improve Capture of Real-Time Data with Achieve® Mapping Catheter
|
|
Jan 15, 2013 Second-Generation Sheath May Facilitate Easier Access to the Inferior Veins When Treating Paroxysmal Atrial Fibrillation
|
|
Aug 24, 2012 Next-Generation Technology Reinforces Safety and Efficacy While Improving Procedure Efficiency
|
|