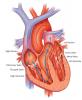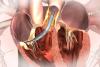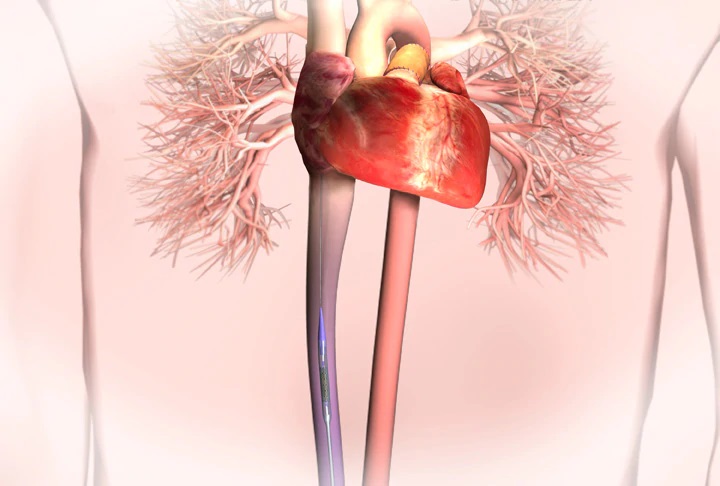
Congenital Heart Valve Disease
Information about the Melody® Transcatheter Pulmonary Valve, which offers a non-surgical valve replacement option for congenital heart disease patients. Melody is the first transcatheter heart valve to receive FDA approval and represents a significant development in the treatment of congenital heart disease.
To learn more, please visit Melody-TPV.com.
The following media kits are made available to provide reporters with background information on the range of Medtronic therapies and the conditions they treat.
MINNEAPOLIS, Jan 25, 2010 (BUSINESS WIRE) -- In a significant development for congenital heart disease patients, Medtronic, Inc. (NYSE: MDT), announced today that its Melody(R) Transcatheter Pulmonary Valve has received U.S. Food and Drug Administration (FDA) approval under a Humanitarian Device Exemption (HDE). This innovative medical device is the first transcatheter heart valve to receive FDA approval.
Delivered through a catheter requiring only a small incision, the Melody valve will benefit children and adults who are born with a malformation of their pulmonary valve, which is the valve between the heart and lungs. These patients often require open-heart surgery to restore effective blood flow to their lungs. Previously, the only way to repair or replace a failed pulmonary valve conduit was through additional surgeries. To date, more than 1,100 patients worldwide have received a Melody valve.
"The Melody Transcatheter Pulmonary Valve is a significant technological breakthrough and offers a reprieve for many patients with congenital heart disease - many of whom are young and will require several heart surgeries over their lifetime," said pediatric cardiologist Dr. William E. Hellenbrand of the NewYork-Presbyterian Morgan Stanley Children's Hospital and Columbia University Medical Center.
"The Melody valve gives patients with congenital heart disease a new, non-surgical approach to managing their disease."
"This novel technology will improve the lives of thousands of patients in the United States," said Dr. John Liddicoat, vice president and general manager of the Structural Heart division, part of the CardioVascular business, at Medtronic. "Medtronic is leading the development of transcatheter therapies for heart valve disease. FDA approval of the Melody Transcatheter Pulmonary Valve is evidence of that leadership."
In October 2006, the Melody valve became the first transcatheter valve to receive regulatory approval anywhere in the world when it received the CE (Conformité Européenne) mark. It is now approved by the FDA for use in the United States under an HDE, a special regulatory approval for treatments intended for fewer than 4,000 U.S. patients per year. HDEs are granted for medical devices that have demonstrated reasonable safety and probable benefit, but not clinical effectiveness.
Medtronic CardioVascular is committed to advancing the treatment of coronary, peripheral, aortic and structural heart disease through collaboration with leading clinicians, researchers and scientists worldwide.
ABOUT MEDTRONIC
Medtronic, Inc. (http://www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology - alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's Annual Report on Form 10-K for the year ended April 24, 2009. Actual results may differ materially from anticipated results.
SOURCE: Medtronic, Inc.
Medtronic, Inc.
Public Relations:
Joe McGrath, 612-819-6421
or
Investor Relations:
Jeff Warren, 763-505-2696