Decision makers in Europe further expand patient access for technology that simplifies diabetes management
Medtronic plc, a global leader in healthcare technology, today announced that reimbursement for its advanced insulin delivery system, MiniMed 780G, has been recently expanded across two more...
Medtronic plc, a global leader in healthcare technology, today announced that reimbursement for its advanced insulin delivery system, MiniMed 780G, has been recently expanded across two more countries in Europe, including Germany and France.
Germany began reimbursing for the new MiniMed™ 780G system[1] with Guardian™ 4 sensor in March while France will begin reimbursement for MiniMed™ 780G system with Guardian™ Sensor 3 mid-April.
Advanced insulin delivery systems include insulin pump therapy combined with continuous glucose monitoring (CGM) systems which provide critical information on glucose levels to help simplify the management of diabetes.
"We welcome decisions of health care authorities across Europe to increase access to advance insulin delivery system technology, making it easier for more people living with diabetes to manage their condition effectively and help improve their quality of life " said Federico Gavioli, Vice President, Diabetes EMEA at Medtronic.
Automated insulin delivery system reimbursement has been expanding across Europe over the past several months. Sweden began reimbursing for the new Guardian™ 4 sensor used with MiniMed™ 780G system. Croatia is now fully reimbursing for the MiniMed 640G system with no limitations and reimburses for the MiniMed 780G system with a copayment. Finally, Estonia started reimbursement for the MiniMedTM 780G system for people under the age of 19.
This announcement follows the recent news about reimbursement decisions for CGM technology for eligible residents living with type 1 diabetes in several countries in North and South America.
About the MiniMedTM 780G system
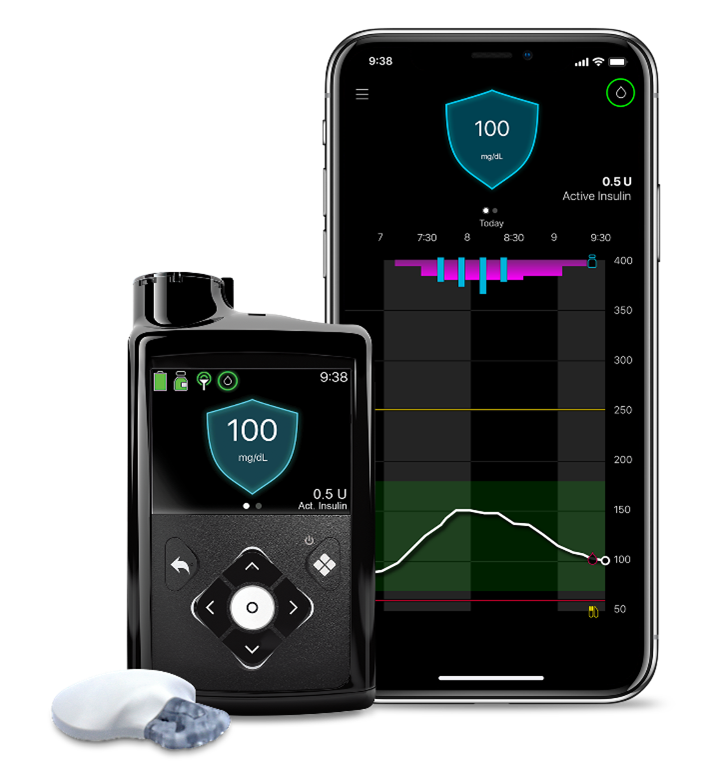
The MiniMedTM 780G system is the most advanced insulin pump system from Medtronic, currently approved for the treatment of type 1 diabetes in people aged 7 years and older. The MiniMedTM 780G system's SmartGuard algorithm (also referred to as the advanced hybrid closed-loop algorithm) automates the delivery of insulin every five minutes — personalizing these doses to auto-correct highs and lows 24 hours a day based on CGM readings[2] [3]. The system enables the personalization of glucose goals with an adjustable target setting as low as 100 mg/dL (5.5 mmol/L). The MiniMedTM 780G system is now available in over 40 countries across Europe, the Middle East and Africa, and is currently being reviewed by the Food and Drug Administration (FDA) for approval in the U.S.
About Continuous Glucose Monitoring (CGM)
CGM systems track glucose levels, every few minutes, 24/7 through a tiny sensor inserted under the skin using an automatic inserter in a location on the body as indicated in the product labeling. The sensor measures interstitial glucose level, which is the glucose found in the fluid between cells. CGM therapy can be used with or without an insulin pump.
About the Diabetes Business at Medtronic (www.medtronicdiabetes.com)
Medtronic is working together with the global community to change the way people manage diabetes. The company aims to transform diabetes care by expanding access, integrating care and improving outcomes, so people living with diabetes can enjoy greater freedom and better health.
Contacts:
Pamela Reese
Public Relations
+1-818-576-3398
[1] The MiniMed™780G pump and new CGM are under FDA and Health Canada review and not currently for sale in the U.S. and Canada.
[2] Carlson, A.L. et al. Safety and glycemic outcomes during the MiniMed™ Advanced Hybrid Closed-Loop system pivotal trial in adolescents and adults with type 1 diabetes. Diab Tech Ther 2021; in press.
[3] Collyns.O. et al Improved Glycemic Outcomes With Medtronic MiniMed Advanced Hybrid Closed-Loop Delivery: Results From a Randomized Crossover Trial Comparing Automated Insulin Delivery With Predictive Low Glucose Suspend in People With Type 1 Diabetes(opens new window). Diab Care 2021, 44: 969-975
See the User Guide for detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events. For further information, contact your local Medtronic representative and/or consult the Medtronic website at medtronic.eu.
