INFUSE® Bone Graft/LT-CAGE® Device
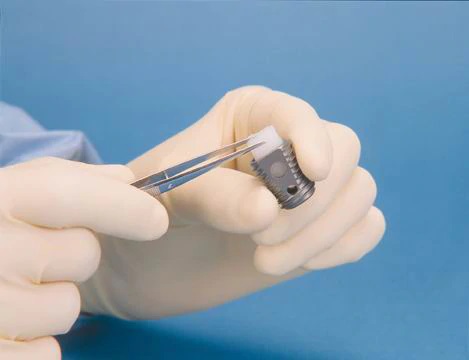
Information about the use of INFUSE® Bone Graft with the LT-CAGE® Lumbar Tapered Fusion Device to treat degenerative disc disease; it's estimated that, in 2002, more than 190,000 Americans will undergo lumbar spinal fusion surgeries to ease their debilitating back pain and get them back on their feet. INFUSE® Bone Graft contains recombinant human bone morphogenetic protein (rhBMP-2), the genetically engineered version of a naturally occurring protein that is capable of initiating bone growth, or bone regeneration, in specific, targeted areas in the spine. Using INFUSE® Bone Graft with the LT-CAGE® device in spine surgery reduces the pain and complications associated with treating degenerative disc disease by eliminating the second surgery required to harvest bone from a patient’s hip, as is done in traditional spinal fusion procedures.
The following media kits are made available to provide reporters with background information on the range of Medtronic therapies and the conditions they treat.