Medtronic receives spine industry’s first and only FDA clearance for breakthrough device indicated for ligament augmentation
Medtronic plc, a global leader in healthcare technology, has received U.S. Food and Drug Administration (FDA) 510(k) clearance and Breakthrough Device designation for its novel LigaPASS™ 2.0 Ligament Augmentation System. LigaPASS™ is the first and only FDA cleared device with indication for ligament augmentation in spine surgery.
Ligament augmentation has been studied for its impact on proximal junctional kyphosis (PJK), a frequent post-operative complication of adult spinal deformity surgery1. While the exact cause is unknown, PJK is thought to involve disruption of spinal ligaments and can affect up to 46% of patients1. The condition can significantly impact a patient’s quality of life.2 The severity of PJK can vary, and some patients may develop a more severe form of the condition called proximal junctional failure (PJF). Patients who develop PJF may develop structural and neurological complications, which increases the need for revision surgery.
“The surgical treatment of adult spinal deformity provides significant clinical benefit to patients but unfortunately many require revision due to early mechanical failure. The most common cause of mechanical failure is proximal junctional kyphosis, “said Christopher Ames, M.D., director of spinal tumor and spinal deformity surgery at UCSF Medical Center in California. “Failure reduction strategies, such as ligament augmentation, are likely to become critical techniques in the treatment of this challenging patient population. In my practice, LigaPASS™ 2.0 helps me meet my goals to reduce revision surgeries with these patients.”
A study of 242 adult spinal deformity cases found patients treated with ligament augmentation had significantly lower PJK and PJF complication rate as follows:
- After one year, the reoperation rate for adult spinal deformity patients treated with ligament augmentation was just 3.3% compared to 15.6% of not treated with ligament augmentation.3,4
The ligament augmentation technique aims to reduce the reoperation rate for PJF and its associated costs, which can be more than $50,000 per procedure5,6
The LigaPASS™ 2.0 system can also be paired with the UNiD™ Adaptive Spine Intelligence (ASI) platform. UNiD™ ASI is transforming the standard of care for patient-specific spine surgery by leveraging data science and artificial intelligence to help surgeons plan, execute and analyze their procedures. LigaPASS™ 2.0 system connectors and bands may also be used in conjunction with the CD Horizon™ Solera™ spinal system.
“This clearance and Breakthrough Device designation demonstrates our ongoing commitment to innovation in spine surgery and delivering industry-leading solutions that improve care for patients and improve the experience for surgeons,” said Dan Wolf, vice president and general manager, intelligent Data Solutions at Medtronic.
Contact:
Kyra Nead
Public Relations
+1-303-886-2549
About LigaPASS™ 2.0 system
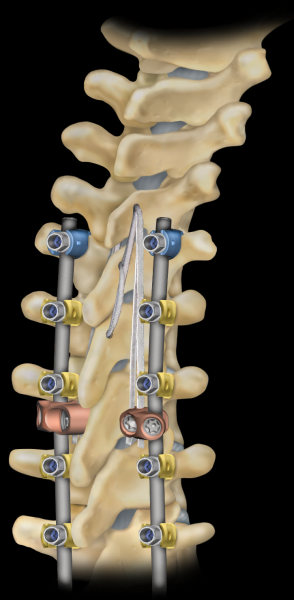
The LigaPASS™ ligament augmentation technique provides surgeons the ability to stabilize between vertebrae that are collapsed or are at risk of collapsing in adult spinal deformity patients requiring constructs that may extend from T1 to the pelvis. This innovative surgical procedure aims to compensate for muscle collapse during open posterior surgery and decrease junctional stress at the UIV and adjacent levels through a band lacing technique using LigaPASS™ 2.0 system implants. The LigaPASS™ 2.0 system medial connector is designed to provide additional support to the junction between the instrumented and adjacent non-instrumented levels.
About UNiD™ ASI Platform
The UNiD ASI platform uses a database of thousands of surgical cases to power algorithms that visualize multiple permutations, allowing surgeons to better understand their patient’s alignment before surgery, customize a surgical plan with highly qualified UNiD™ LAB biomedical engineers and use a patient-specific rod industrially bent in the optimal plane to help ensure the goals of the surgery are achieved.
About the CD Horizon™ Solera™ Spinal Systems
These systems help provide immobilization and stabilization of spinal segments. The systems consist of a variety of screws and other implant components that can be rigidly locked into a variety of configurations, with each construct being tailor-made for the patient's unique anatomy and sagittal alignment needs.
References
- Lau, Darryl, et al. "Proximal junctional kyphosis and failure after spinal deformity surgery: a systematic review of the literature as a background to classification development." Spine 39.25 (2014): 2093-2102.
- Lau, Darryl, et al. "The clinical correlation of the Hart-ISSG Proximal Junctional Kyphosis Severity Scale with health-related quality-of-life outcomes and need for revision surgery." Spine 41.3 (2016): 213-223.
- Safaee MM, Deviren V, Dalle Ore CL, Scheer JK, Lau D, Osario J, Nicholls F, Ames CP. Ligament augmentation for prevention of proximal junctional kyphosis and proximal junctional failure in adult spinal deformity. J Neurosurg Spine. 2018 Feb 23
- Safaee MM, Haddad AF, Fury M, Maloney PR et al. Reduced proximal junctional failure with ligament augmentation in adult spinal deformity: a series of 242 cases with a minimum 1-year follow-up. J Neurosurg Spine. 2021 Aug 20;1-9.
- Theologis, AA, Miller L, Callahan M, et al. Economic Impact of Revision Surgery for Proximal Junctional Failure After Adult Spinal Deformity Surgery: A Cost Analysis of 57 Operations in a 10-year Experience at a Major Deformity Center. Spine (Phila Pa 1976). 2016 Aug 15;41(16):E964-E972.
- Safaee MM, Dalle Ore CL, Zygourakis CC, Deviren V, Ames CP. The unreimbursed costs of preventing revision surgery in adult spinal deformity: analysis of cost-effectiveness of proximal junctional failure prevention with ligament augmentation. Neurosurg Focus. 2018 May;44(5):E13.
UC202302160 EN