Sep 18, 2020
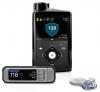
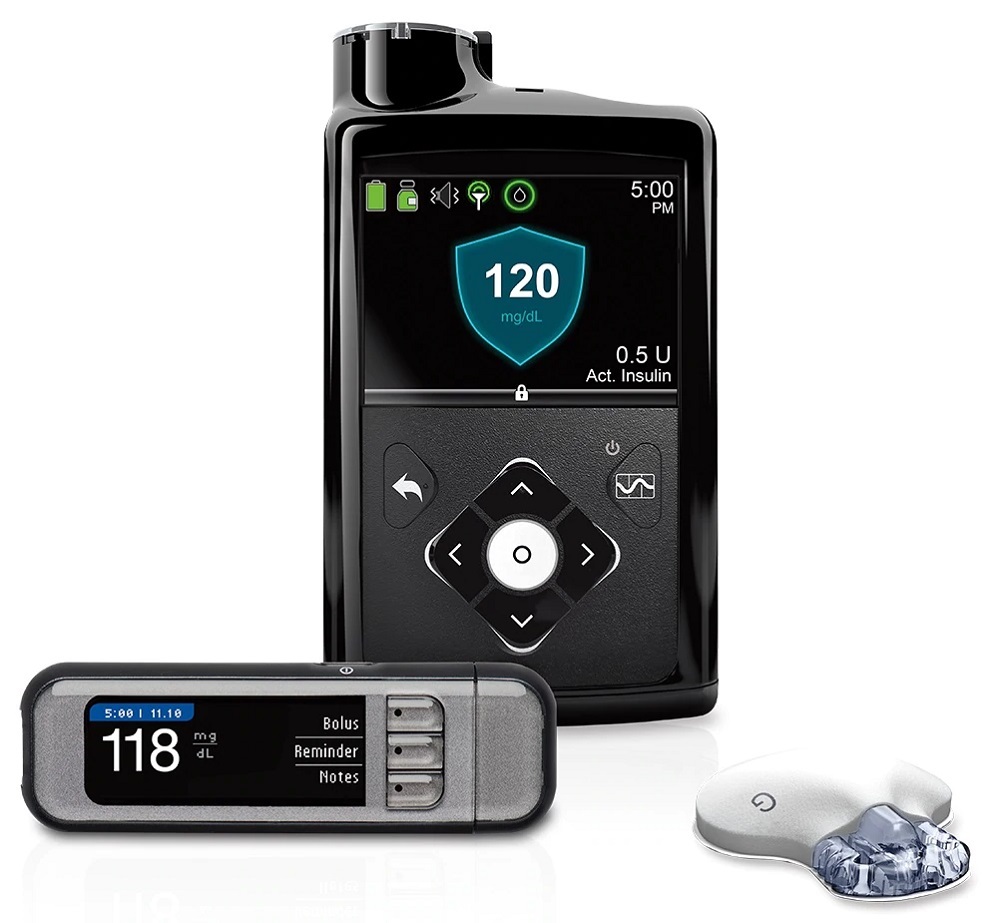
MiniMed® 670G Hybrid Closed Loop System
Information about the MiniMed® 670G system, the world’s first hybrid closed loop system for individuals with type 1 diabetes.
The following media kits are made available to provide reporters with background information on the range of Medtronic therapies and the conditions they treat.
May 28, 2019
|
|
Jun 24, 2018
|
|
Jun 21, 2018
|
|
Jun 7, 2017 The MiniMed(TM) 670G System Features the Company's Most Advanced SmartGuard(TM)HCL Technology and New Guardian(TM) Sensor 3
|
|
Sep 28, 2016 The MiniMed® 670G System Features the Company's Most Advanced SmartGuard(TM) Algorithm To-Date
|
|