New Medtronic RespArray™ patient monitor aims to reduce risk of respiratory compromise and improve workflow
Medtronic plc, a global leader in healthcare technology, announces U.S. Food and Drug Administration (FDA) 510(k) clearance for the RespArray™ patient monitor designed for procedural sedation and medical-surgical units.
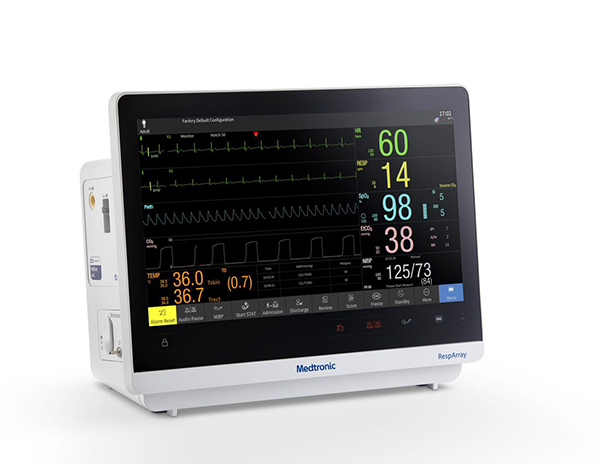
RespArray™ is engineered with market leading technologies, Nellcor™ pulse oximetry and Microstream™ capnography, as well as electrocardiogram (ECG), non-invasive blood pressure (NiBP), and temperature monitoring. Medtronic is planning a limited market release in late Q4 2022 followed by a full market release in early 2023.
Patient safety is every clinician’s top priority. Due to the pandemic, many patients in procedural sedation and medical-surgical units require more attention than ever before. But staffing shortages and clinician burnout have led to a decrease in patient safety.1
The RespArrayTM patient monitor — with NellcorTM pulse oximetry and MicrostreamTM capnography — offers clinicians a new powerful choice in monitoring solutions. Designed with smart algorithms, it helps detect respiratory compromise early and reduce alarm fatigue. Microstream™ capnography reduces unnecessary nuisance alarms by 53%.2
Respiratory compromise, a decline in respiratory function, is a common cause of preventable harm and death.3,4 In medical-surgical units, patients on opioids — who are at high risk for respiratory compromise — are commonly only monitored with spot checking. With better continuous monitoring and response, 97% of postoperative opioid-induced respiratory depression is preventable.5 Implementing continuous monitoring of high-risk patients on opioids on medical-surgical units supports patient safety and may help save median-sized hospitals $535,000 annually.6
The RespArray™ patient monitor also features simple connectivity and seamlessly integrates into the hospital’s EMR to improve workflow. For clinicians this means less time manually charting and more time to focus on what matters — caring for patients.
“Helping to keep patients safe is our top priority,” said Frank Chan, president of the Patient Monitoring business, which is part of the Medical Surgical Portfolio at Medtronic. “We engineered RespArray™ to expand access in procedural sedation and medical-surgical units to critical monitoring technologies that are always watching out for patients. These technologies can help clinicians detect respiratory compromise early so they can intervene fast.”
For more details and media assets, please visit our media kit.
Contacts:
Tammy Hudson Ryan Weispfenning
Public Relations Investor Relations
+1-678-488-5337 +1-763-505-4626
References:
- Emergency Care Research Institute (ECRI). 2022 Top 10 Patient Safety Concerns. Special Report. March 2022.
- Internal test data on file.
- Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141.
- Ochroch EA, Russell MW, Hanson WC, 3rd, et al. The impact of continuous pulse oximetry monitoring on intensive care unit admissions from a postsurgical care floor. Anesthesia and analgesia. 2006;102(3):868-875.
- Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659-665.
- Khanna A, Junquist C, Buhre W, Soto R, di Piazza F, Saager L. Modeling the Cost Savings of Continuous Pulse Oximetry and Capnography Monitoring of United States General Care Floor Patients Receiving Opioids Based on the PRODIGY Trial. Adv Ther. 2021 May 24:1–15. doi: 10.1007/s12325-021-01779-7.
The RespArray™ patient monitor should not be used as the sole basis for diagnosis or therapy and is intended only as an adjunct in patient assessment.
