Month Year
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
Month Year
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Sep 7, 2022
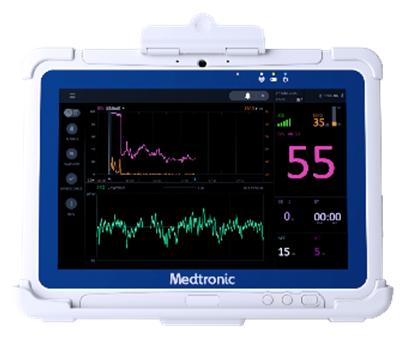
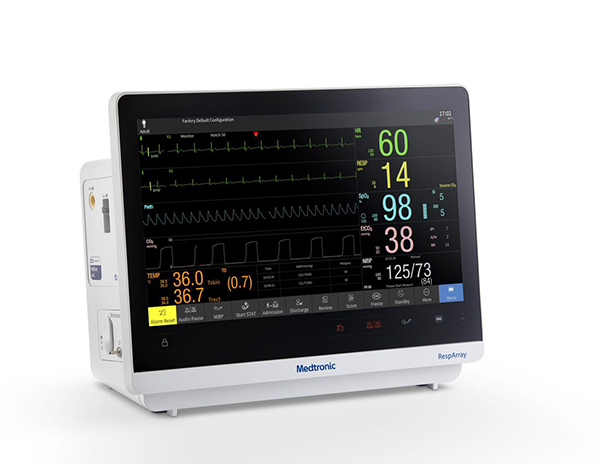
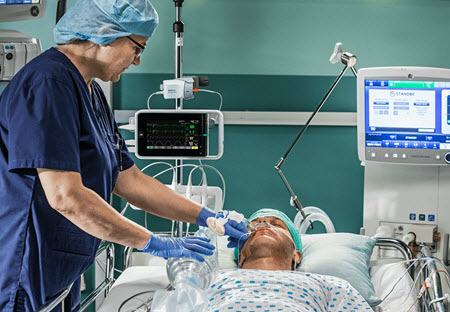
Medtronic plc, a global leader in healthcare technology, announces U.S. Food and Drug Administration (FDA) 510(k) clearance for the RespArray™ patient monitor designed for procedural sedation and medical-surgical units.
-
Aug 31, 2022
Partnership enables Patient Monitoring business’ commitment to transforming health monitoring by expanding access to continuous vital sign monitoring of patients from in-hospital to home
-
Jun 1, 2022
Clinicians now have added ability to personalize care with the integration of two continuous monitoring solutions from Medtronic plc...