Replacing Aortic Heart Valves Without Open-Heart Surgery
In seemingly perfect shape on the surface, 26-year-old Andrew “AJ” Jones went from being a fitness model to fighting for his life.
Singing has always been central to Ron Hargrave’s life. As a young man he even went to Hollywood to write songs and perform. But at age 89, he began feeling too tired to walk more than half a block. And worse for Ron, he couldn’t draw enough breath to sing.
“I was going down the tubes,” he said. “Old man time was moving in.”
Doctors diagnosed him with severe aortic stenosis, a narrowing of the aortic heart valve that obstructs blood flow and forces the heart to work harder to pump blood to the body. Without a new valve, Ron could die.
Typically, heart valve replacement involves invasive open-heart surgery – cutting open the chest and cutting through the breastbone – then removing the diseased valve and sewing in a new, artificial valve. But due to Ron’s age and weakness, and the fact that he had undergone an earlier open-heart surgery for a different problem, doctors considered him a high-risk patient who might not survive another open-heart surgery.
'You have to see Mr. Hargrave. He's been singing.'
Mary Schoenbaum, RN, ACNP, valve clinic coordinator
“Ron’s symptoms were rather advanced. We thought a CoreValve device would be an excellent option,” said cardiothoracic surgeon Mark Cunningham.
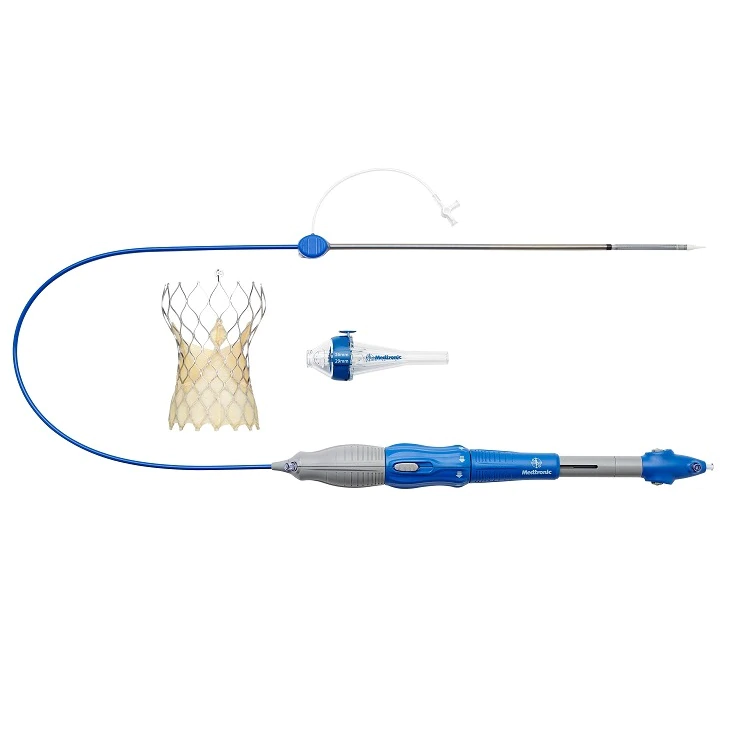
The CoreValve Evolut TAVR System is a self-expandable valve inserted into the heart without open-heart surgery. The valve is squeezed down to roughly the width of a pencil, and then inserted into the body, typically through a vein in the groin or upper chest. It’s then guided into the heart and deployed, where it expands to take over for the diseased valve.
The procedure is known as transcatheter aortic valve replacement (TAVR). (Watch a video to see how the CoreValve™ Evolut™ TAVR platform works.)
“When I walked into the unit the following day, all of the nurses came up to me and said ‘you have to go and see Mr. Hargrave. He’s been singing,’” said Mary Schoenbaum, RN, ACNP and the valve clinic coordinator.
The Food and Drug Administration (FDA) originally approved the minimally-invasive device for cases like Ron’s – where patients are so sick or so frail they are at high-risk of not surviving open-heart surgery. But in July of 2017, the FDA approved the CoreValve Evolut platform’s use for even more patients, to include those considered at intermediate risk of dying during open-heart surgery.
“At Medtronic, we take pride in our ability to generate meaningful clinical evidence that founds our products and therapies in strong science,” said Pieter Kappetein, M.D., Ph.D., vice president, chief medical officer of the Structural Heart business, a part of Medtronic’s Cardiac and Vascular Group. “We continue to invest in clinical trials, the results of which will help shape the future of cardiac care, especially in the heart valve space.”
A Medtronic-sponsored non-inferiority study called SURTAVI found as secondary endpoints that intermediate-risk patients treated with the CoreValve system recovered faster, left the hospital sooner and suffered fewer post-procedure strokes than patients who underwent open-heart surgery.
Ron, who was considered high-risk at the time of his procedure, was already feeling well enough in the Intensive Care Unit to give his nurses a thumbs-up. Today the life-long musician sings the praises of CoreValve. “It brought my life back as it was.” (Watch more of Ron’s story.)
Important Safety Information
INDICATIONS The Medtronic CoreValve™ Evolut™ R and CoreValve™ Evolut™ PRO systems are indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be at intermediate or greater risk for open surgical therapy (i.e., Society of Thoracic Surgeons [STS] predicted risk of operative mortality score ≥3% at 30 days and other clinical comorbidities unmeasured by the STS risk calculator).
The Medtronic CoreValve™ Evolut™ R and CoreValve™ Evolut™ PRO system are indicated for use in patients with symptomatic heart disease due to failure (stenosed, insufficient, or combined) of a surgical bioprosthetic aortic valve who are judged by a heart team, including a cardiac surgeon, to be at high or greater risk for open surgical therapy (i.e., STS predicted risk of operative mortality score ≥8% or at a ≥15% risk of mortality at 30 days).
CONTRAINDICATIONS The CoreValve Evolut R and PRO systems are contraindicated for patients presenting with any of the following conditions: known hypersensitivity or contraindication to aspirin, heparin (HIT/HITTS) and bivalirudin, ticlopidine, clopidogrel, Nitinol (Titanium or Nickel), or sensitivity to contrast media, which cannot be adequately premedicated; ongoing sepsis, including active endocarditis; preexisting mechanical heart valve in the aortic position.
WARNINGS General Implantation of the CoreValve Evolut R and PRO systems should be performed only by physicians who have received Medtronic CoreValve training. This procedure should only be performed where emergency aortic valve surgery can be performed promptly. Mechanical failure of the delivery catheter system and/or accessories may result in patient complications. Accelerated deterioration of the bioprosthesis may occur in patients presenting with an altered calcium metabolism.
PRECAUTIONS General The safety and effectiveness of the CoreValve Evolut R and PRO systems have not been evaluated in the pediatric population. The safety and effectiveness of the bioprosthesis for aortic valve replacement have not been evaluated in the following patient populations: patients who do not meet the criteria for symptomatic severe native aortic stenosis as defined: (1) symptomatic severe high gradient aortic stenosis — aortic valve area ≤1.0 cm2 or aortic valve area index ≤0.6 cm2m2, a mean aortic valve gradient ≥40 mm Hg; or a peak aortic-jet velocity ≥4.0 m/s, (2) symptomatic severe low-flow, low-gradient aortic stenosis — aortic valve area ≤1.0cm2 or aortic valve area index ≤0.6 cm2/m2, a mean aortic valve gradient <40 mmHg; and a peak aortic-jet velocity <4.0 m/s ; who are at low surgical risk (predicted perioperative mortality risk of <3%); with untreated, clinically significant coronary artery disease requiring revascularization; with a pre-existing prosthetic heart valve with a rigid support structure in either the mitral or pulmonic position if either the pre-existing prosthetic heart valve could affect the implantation or function of the bioprosthesis or the implantation of the bioprosthesis could affect the function of the pre-existing prosthetic heart valve; with cardiogenic shock manifested by low cardiac output, vasopressor dependence, or mechanical hemodynamic support. The safety and effectiveness of a CoreValve Evolut R and PRO bioprosthesis implanted within a failed pre-existing transcatheter bioprosthesis has not been demonstrated. Implanting a CoreValve Evolut R or PRO bioprosthesis in a degenerated surgical bioprosthesis [transcatheter aortic valve in surgical aortic valve (TAV in SAV)] should be avoided in the following conditions. The degenerated surgical bioprosthesis presents with: a significant concomitant perivalvular leak (between the prosthesis and the native annulus), is not securely fixed in the native annulus, or is not structurally intact (e.g., wireform frame fracture); partially detached leaflet that in the aortic position may obstruct a coronary ostium; stent frame with a manufacturer’s labeled inner diameter <17 mm. The safety and effectiveness of the bioprosthesis for aortic valve replacement have not been evaluated in patient populations presenting with the following: blood dyscrasias as defined: leukopenia (WBC <1000 cells/mm3), thrombocytopenia (platelet count <50,000 cells/mm3), history of bleeding diathesis or coagulopathy, or hypercoagulable states; congenital bicuspid or unicuspid valve; mixed aortic valve disease (aortic stenosis and aortic regurgitation with predominant aortic regurgitation [3-4+]); moderate to severe (3-4+) or severe (4+) mitral or severe (4+) tricuspid regurgitation; hypertrophic obstructive cardiomyopathy; new or untreated echocardiographic evidence of intracardiac mass, thrombus, or vegetation; native aortic annulus size <18 mm or >30 mm for CoreValve Evolut R and <18 mm or >26 mm for CoreValve Evolut PRO per the baseline diagnostic imaging or surgical bioprosthetic aortic annulus size <17 mm or >30 mm for CoreValve Evolut R and <17 mm or >26 mm for CoreValve Evolut PRO; transarterial access not able to accommodate an 18 Fr sheath or the 14 Fr equivalent EnVeo™ R InLine sheath when using Model ENVEOR-US or transarterial access not able to accommodate a 20 Fr introducer sheath or the 16 Fr equivalent EnVeo™ R InLine sheath when using Model ENVEOR-N-US; sinus of valsalva anatomy that would prevent adequate coronary perfusion; moderate to severe mitral stenosis; severe ventricular dysfunction with left ventricular ejection fraction (LVEF) <20%; symptomatic carotid or vertebral artery disease; severe basal septal hypertrophy with an outflow gradient.
Prior to Use Exposure to glutaraldehyde may cause irritation of the skin, eyes, nose, and throat. Avoid prolonged or repeated exposure to the vapors. Damage may result from forceful handling of the catheter. Prevent kinking of the catheter when removing it from the packaging. This device was designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death. The bioprosthesis size must be appropriate to fit the patient’s anatomy. Proper sizing of the device is the responsibility of the physician. Refer to Instructions for Use for available sizes. Failure to implant a device within the sizing matrix could lead to adverse effects such as those listed below. Patients must present with access vessel diameters of ≥5 mm when using Model ENVEOR-US or ≥5.5 mm when using Model ENVEOR-N-US, or patients must present with an ascending aortic (direct aortic) access site ≥60 mm from the basal plane for both systems. Implantation of the bioprosthesis should be avoided in patients with aortic root angulation (angle between plane of aortic valve annulus and horizontal plane/vertebrae) of >30° for right subclavian/axillary access or >70° for femoral and left subclavian/axillary access. Use caution when using the subclavian/axillary approach in patients with a patent LIMA graft or patent RIMA graft. For direct aortic access, ensure the access site and trajectory are free of patent RIMA or a pre-existing patent RIMA graft.
During Use For direct aortic and subclavian access procedures, care must be exercised when using the tip-retrieval mechanism to ensure adequate clearance to avoid advancement of the catheter tip through the bioprosthesis leaflets during device closure. For direct aortic access procedures, use a separate introducer sheath; do not use the EnVeo R InLine sheath. Adequate rinsing of the bioprosthesis with sterile saline, as described in the Instructions for Use, is mandatory before implantation. During rinsing, do not touch the leaflets or squeeze the bioprosthesis. If a misload is detected, unsheath the bioprosthesis and examine the bioprosthesis for damage (for example, permanent frame deformation, frayed sutures, or valve damage). Do not attempt to reload a damaged bioprosthesis. Do not load the bioprosthesis onto the catheter more than two times or after it has been inserted into a patient. Use the deployment knob to deploy and recapture the bioprosthesis. Do not use the trigger for deploying or recapturing because it could cause inaccurate placement of the bioprosthesis. Once the radiopaque capsule marker band reaches the distal end of the radiopaque paddle attachment (point of no recapture), retrieval of the bioprosthesis from the patient is not recommended. Retrieval after the point of no recapture may cause mechanical failure of the delivery catheter system, aortic root damage, coronary artery damage, myocardial damage, vascular complications, prosthetic valve dysfunction (including device malposition), embolization, stroke, and/or emergent surgery. During deployment, the bioprosthesis can be advanced or withdrawn as long as annular contact has not been made. Once annular contact is made, the bioprosthesis cannot be advanced in the retrograde direction; recapture until the bioprosthesis is free from annular contact, and then reposition in the retrograde direction. If necessary, and the radiopaque capsule marker band has not yet reached the distal end of the radiopaque paddle attachment, the bioprosthesis can be withdrawn (repositioned) in the antegrade direction. However, use caution when moving the bioprosthesis in the antegrade direction. While the catheter is in the patient, ensure the guidewire is extending from the tip. Do not remove the guidewire from the catheter while the catheter is inserted in the patient. Use the handle of the delivery system to reposition the bioprosthesis. Do not use the outer catheter sheath. There will be some resistance when the catheter is advanced through the vasculature. If there is a significant increase in resistance, stop advancement and investigate the cause of the resistance (for example, magnify the area of resistance) before proceeding. Do not force passage. Forcing passage could increase the risk of vascular complications (for example, vessel dissection or rupture). Persistent force on the catheter can cause the catheter to kink which could increase the risk of vascular complications (for example, vessel dissection or rupture).Once deployment is complete, repositioning of the bioprosthesis is not recommended. Repositioning of a deployed valve may cause aortic root damage, coronary artery damage, myocardial damage, vascular complications, prosthetic valve dysfunction (including device malposition), embolization, stroke, and/or emergent surgery. Do not attempt to retrieve or to recapture a bioprosthesis if any one of the outflow struts is protruding from the capsule. If any one of the outflow struts has deployed from the capsule, the bioprosthesis must be released from the catheter before the catheter can be withdrawn. Ensure the capsule is closed before catheter removal. When using a separate introducer sheath, if increased resistance is encountered when removing the catheter through the introducer sheath, do not force passage. Increased resistance may indicate a problem and forced passage may result in damage to the device and/or harm to the patient. If the cause of resistance cannot be determined or corrected, remove the catheter and introducer sheath as a single unit over the guidewire, and inspect the catheter and confirm that it is complete. Clinical long-term durability has not been established for the bioprosthesis. Evaluate bioprosthesis performance as needed during patient follow-up. Post procedure, administer appropriate antibiotic prophylaxis as needed for patients at risk for prosthetic valve infection and endocarditis. Post procedure, administer anticoagulation and/or antiplatelet therapy per physician/clinical judgment. Excessive contrast media may cause renal failure. Pre procedure, measure the patient’s creatinine level. During the procedure, monitor contrast media usage. Conduct the procedure under fluoroscopy. The safety and efficacy of a CoreValve Evolut R or CoreValve Evolut PRO bioprosthesis implanted within a transcatheter bioprosthesis have not been demonstrated. However, in the event that a CoreValve Evolut R or CoreValve Evolut PRO bioprosthesis must be implanted within a transcatheter bioprosthesis to improve valve function, valve size and patient anatomy must be considered before implantation of the CoreValve Evolut R or CoreValve Evolut PRO bioprosthesis to ensure patient safety (for example, to avoid coronary obstruction). In the event that valve function or sealing is impaired due to excessive calcification or incomplete expansion, a post-implant balloon dilatation of the bioprosthesis may improve valve function and sealing. To ensure patient safety, valve size and patient anatomy must be considered when selecting the size of the balloon used for dilatation. The balloon size chosen for dilatation should not exceed the diameter of the native aortic annulus or, for surgical bioprosthetic valves, the manufacturer’s labeled inner diameter. Refer to the specific balloon catheter manufacturer's compliance chart to ensure that the applied inflation pressure does not result in a balloon diameter that exceeds the indicated annulus range for the bioprosthesis. Refer to the specific balloon catheter manufacturer’s labeling for proper instruction on the use of balloon catheter devices.
Note: Bench testing has only been conducted to confirm compatibility with NuMED Z-MEDTMand Z-MED IITM Balloon Aortic Valvuloplasty catheters where CoreValve Evolut R and CoreValve Evolut PRO bioprosthesis device performance was maintained after dilation. Data on File.
POTENTIAL ADVERSE EVENTS Potential risks associated with the implantation of the CoreValve Evolut R or CoreValve Evolut PRO transcatheter aortic valve may include, but are not limited to, the following: • death • myocardial infarction, cardiac arrest, cardiogenic shock, cardiac tamponade • coronary occlusion, obstruction, or vessel spasm (including acute coronary closure) • cardiovascular injury (including rupture, perforation, tissue erosion, or dissection of vessels, ascending aorta trauma, ventricle, myocardium, or valvular structures that may require intervention) • emergent surgical or transcatheter intervention (for example, coronary artery bypass, heart valve replacement, valve explant, percutaneous coronary intervention [PCI], balloon valvuloplasty) • prosthetic valve dysfunction (regurgitation or stenosis) due to fracture; bending (out-of-round configuration) of the valve frame; underexpansion of the valve frame; calcification; pannus; leaflet wear, tear, prolapse, or retraction; poor valve coaptation; suture breaks or disruption; leaks; mal-sizing (prosthesis-patient mismatch); malposition (either too high or too low)/malplacement • prosthetic valve migration/embolization • prosthetic valve endocarditis • prosthetic valve thrombosis • delivery catheter system malfunction resulting in the need for additional re-crossing of the aortic valve and prolonged procedural time • delivery catheter system component migration/embolization • stroke (ischemic or hemorrhagic), transient ischemic attack (TIA), or other neurological deficits • heart failure • cardiac failure or low cardiac output • ancillary device embolization • individual organ (for example, cardiac, respiratory, renal [including acute kidney failure]) or multi-organ insufficiency or failure • major or minor bleeding that may require transfusion or intervention (including life-threatening or disabling bleeding) • vascular access-related complications (e.g., dissection, perforation, pain, bleeding, hematoma, pseudoaneurysm, irreversible nerve injury, compartment syndrome, arteriovenous fistula, stenosis) • mitral valve regurgitation or injury • conduction system disturbances (for example, atrioventricular node block, left-bundle branch block, asystole), which may require a permanent pacemaker • infection (including septicemia) • hypotension or hypertension • hemolysis • peripheral ischemia • bowel ischemia • abnormal lab values (including electrolyte imbalance) • allergic reaction to antiplatelet agents, contrast medium, or anesthesia • exposure to radiation through fluoroscopy and angiography • permanent disability.
Please reference the CoreValve Evolut R and CoreValve Evolut PRO Instructions for Use for more information regarding indications, warnings, precautions, and potential adverse events.
CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician.