FDA Approves Next Generation Medtronic Leadless Pacemaker
Micra AV uses a novel approach and the most advanced pacing algorithms in Medtronic history.
Jan. 21, 2020: Medtronic is making pacemaker history – again. The Food and Drug Administration (FDA) today approved Micra™ AV, Medtronic’s next generation of the world’s smallest pacemaker.
The decision means many more patients who need pacing may now be candidates to receive that therapy from a leadless pacemaker.
“The word that comes to mind is transformational,” said Robert Kowal, M.D., Vice President of Medical Affairs and Chief Medical Officer of Cardiac Rhythm and Heart Failure (CRHF) at Medtronic. “Technology changes to do two things. One, make the therapy better and two, make the therapy safer. This is about both of those things,” he said.
FDA approval of Micra AV expands the number of people who can receive leadless pacemaker therapy.
Until now, treatment with leadless pacing was appropriate in only about 15 percent of pacemaker cases. But because of its unique way of sensing and then pacing, Micra AV can treat patients with AV block, a condition in which the electrical signals between the chambers of the heart (the atria and the ventricle) are impaired. With the approval of Micra AV, Medtronic’s leadless pacing technology is now available to approximately half of all pacemaker patients in the U.S.
“It’s very exciting to be able to bring new technology to more patients,” said Kowal. “That’s the core of the Medtronic Mission. And this is just the beginning. Even though this is the next generation of micro technology, I still see this field as being in its infancy. There’s still a lot more to do.”
Never Been Done Before
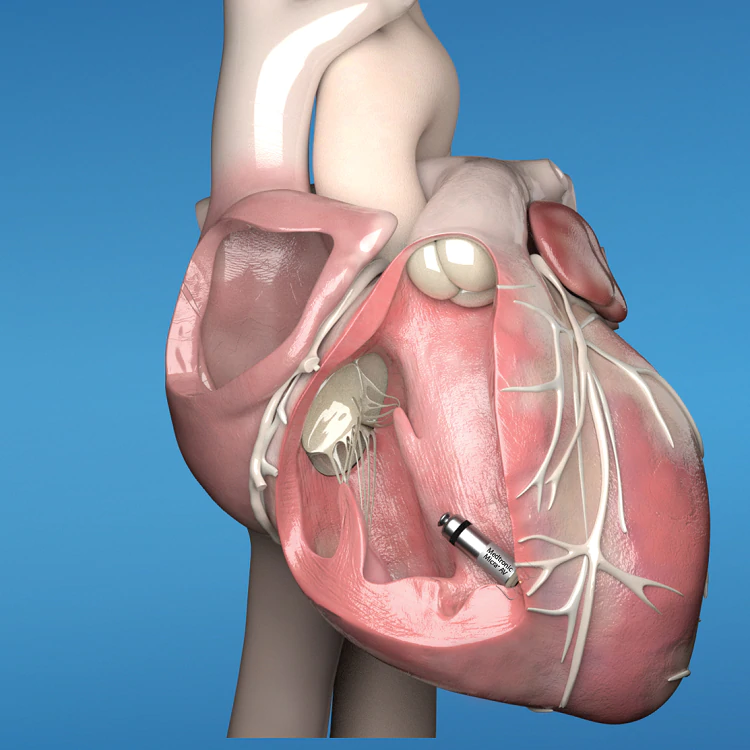
Micra AV is unique in the history of pacemakers. Until now, pacemakers sensed the heart’s electrical signals and used that information to provide pacing. But Medtronic scientists figured out how to re-purpose sensors, already inside the original Micra pacemaker, to recognize certain physical, or mechanical movements, within the heart – an approach to pacing that’s never been done before. Micra AV can detect the mechanical movement of a beat in one chamber of the heart – the atrium – and then pace another chamber where Micra is implanted – the ventricle – so the atrium and ventricle beat in synchrony.
Dr. Larry Chinitz, Director of the Heart Rhythm Center at NYU Langone Health, implanted the very first Micra pacemaker in the United States in 2014 and recently led the MARVEL 2 clinical study of Micra AV. Results from the study , presented at the American Heart Association 2019 Scientific Sessions and published simultaneously in JACC: Clinical Electrophysiology, showed that mechanical atrial sensing using Micra AV was capable of providing AV synchrony in patients with AV block and normal sinus rhythm. “We are now able to achieve AV synchrony with a device the size of a pill, placed in the ventricular apex without incisions, without leads and a 63 percent lower incidence of complications,” said Dr. Chinitz. “If this is not a game changer, if this is not the future of cardiac pacing, I have never witnessed such an event in my career.”
Most Technically Complex Pacing Algorithms in Medtronic History
The series of 11 algorithms which sense the atrial heartbeat and then trigger a corresponding beat in the ventricle are the key to Micra AV, and they’re unlike anything ever developed at Medtronic.
“It’s quite complex,” said Todd Sheldon, Distinguished Scientist in CRHF. “It’s probably more complex detection from a pacing perspective of any algorithm that we’ve done in the history of Medtronic, as far as pure beat-to-beat pacing algorithms go.”
In their early stages, the algorithms, developed by Sheldon and a team of Medtronic scientists, were so complicated that they quickly drained Micra’s battery power. An engineering team led by systems engineer Julie Pronovici tackled that issue by adding firmware to run the algorithms, re-designing Micra’s circuitry to make its algorithm processing more efficient and adding software to be able to program and optimize the system.
“This is by far the most innovative, most technically challenging product project I’ve ever been involved with in my 19 years at Medtronic,” Pronovici said.
If this is not a game changer, if this is not the future of cardiac pacing, I have never witnessed such an event in my career.
Dr. Larry Chinitz, Director of the Heart Rhythm Center at New York University’s Langone Health
"This is the Future"
The FDA approved the original Micra leadless pacemaker in the United States in 2016. Since then, more than 50,000 patients in 60 countries have received the device.
Studies have shown leadless pacing provides significant benefit to patients. Traditional pacemakers require surgery to create a pocket in the upper chest for the battery, and wires (leads) run from the battery into the heart. Leadless pacemakers are implanted inside the heart through the femoral vein in a minimally invasive procedure. They require no leads and studies have shown a 63 percent reduction in complications as compared to traditional pacemakers.
“One of my favorite words is ‘disruptive,’” said Dr. Chinitz. “Because ‘disruptive’ means you change the way physicians practice. You change the way people think. That’s what leadless pacing does. It’s a huge, dramatic change and I really believe this is the future."
Important Safety Information
Indications (or Intended Use)
Micra devices, Micra Model MC1VR01 and Micra AV Model MC1AVR1, are indicated for use in patients who have experienced one or more of the following conditions:
• Paroxysmal or permanent high-grade AV block in the presence of AF
<
• Paroxysmal or permanent high-grade AV block in the absence of AF, as an alternative to dual chamber pacing, when a dual-chamber transvenous pacing system is considered difficult, high risk, or not deemed necessary for effective therapy
• Symptomatic bradycardia-tachycardia syndrome or sinus node dysfunction (sinus bradycardia or sinus pauses), as an alternative to atrial or dual chamber pacing, when a dual-chamber transvenous pacing system is considered difficult, high risk, or not deemed necessary for effective therapy
Micra AV Model MC1AVR1 is also indicated for VDD pacing in patients with adequate sinus rates who may benefit from maintenance of AV synchrony. The Micra AV device provides AV synchronous ventricular pacing similar to a transvenous VDD system. The implanted device depends on the appropriate sensing of atrial mechanical signals to achieve AV synchrony. The level of AV synchrony may vary in individual patients and may not be predictable prior to implant.
Rate-responsive pacing is indicated to provide increased heart rate appropriate to increasing levels of activity.
The device is designed to be used only in the right ventricle.
Contraindications
Micra Model MC1VR01 and Micra AV Model MC1AVR1 are contraindicated for patients who have the following types of medical devices implanted: an implanted device that would interfere with the implant of the Micra device in the judgment of the implanting physician, an implanted inferior vena cava filter, a mechanical tricuspid valve, or an implanted cardiac device providing active cardiac therapy that may interfere with the sensing performance of the Micra device.
The device is contraindicated for patients who have the following conditions: femoral venous anatomy unable to accommodate a 7.8 mm (23 French) introducer sheath or implant on the right side of the heart (for example, due to obstructions or severe tortuosity), morbid obesity that prevents the implanted device from obtaining telemetry communication within ≤12.5 cm (4.9 in), or known intolerance to the materials listed in the Instruction for Use, or to heparin, or sensitivity to contrast media that cannot be adequately premedicated, or if the steroid dose from this device cannot be tolerated.
Warnings and Precautions
End of Service (EOS) – When the EOS condition is met, the clinician has the option of permanently programming the device to Off and leaving it in the heart, or retrieving the device, provided the device has not yet become encapsulated. Removal of the Micra device after it has become encapsulated may be difficult because of the development of fibrotic tissue. If removal of the device is required, it is recommended that the removal be performed by a clinician who has expertise in the removal of implanted leads.
MRI conditions for use – Before an MRI scan is performed on a patient implanted with the Micra device, the cardiology and radiology professionals involved in this procedure must understand the requirements specific to their tasks as defined in the device manuals.
Rate-responsive mode may not be appropriate for patients who cannot tolerate pacing rates above the programmed Lower Rate. For Micra Model MC1VR01, asynchronous VVIR pacing with sinus rhythm may not be appropriate when competitive pacing is considered undesirable or causes symptoms of pacemaker syndrome. The patient’s age and medical condition should be considered by physicians and patients as they select the pacing system, mode of operation, and implant technique best suited to the individual.
Precautions should be taken before administering anticoagulant agents, antiplatelet agents, or contrast media in patients with known hypersensitivity to these agents.
The use of deactivated Micra devices in situ and an active Micra device, or an active transvenous pacemaker or defibrillator, has not been clinically tested to determine whether EMI or physical interaction is clinically significant. Bench testing supports that implantation of an active Micra device, or an active transvenous pacemaker or defibrillator, next to an inactivated Micra device is unlikely to cause EMI or physical interaction. Post-approval studies are planned to characterize risks of co-implanted, deactivated Micra devices. Currently recommended end of device life care for a Micra device may include the addition of a replacement device with or without explanation of the Micra device, which should be turned off.
For Micra AV Model MC1AVR1, patient activities and environments which present mechanical vibrations to the patient can interfere with the mechanical sensing of atrial contractions. This can result in a loss of AV synchrony.
Potential Adverse Events or Potential Complications
Potential complications include, but are not limited to, toxic/allergic reaction, oversensing, pacemaker syndrome, cardiac arrest, acceleration of tachycardia, necrosis, myocardial infarction and surgical complications such as cardiac perforation, pericardial effusion, cardiac tamponade, device embolization, hematoma, AV fistula, vessel dissection, infection, cardiac inflammation, and thrombosis.
See the device manuals for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, MRI conditions for use, and potential complications/adverse events. For further information, please call Medtronic at 1-800-328-2518 and/or consult Medtronic’s website at www.medtronic.com.
Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician.
L001-01172020