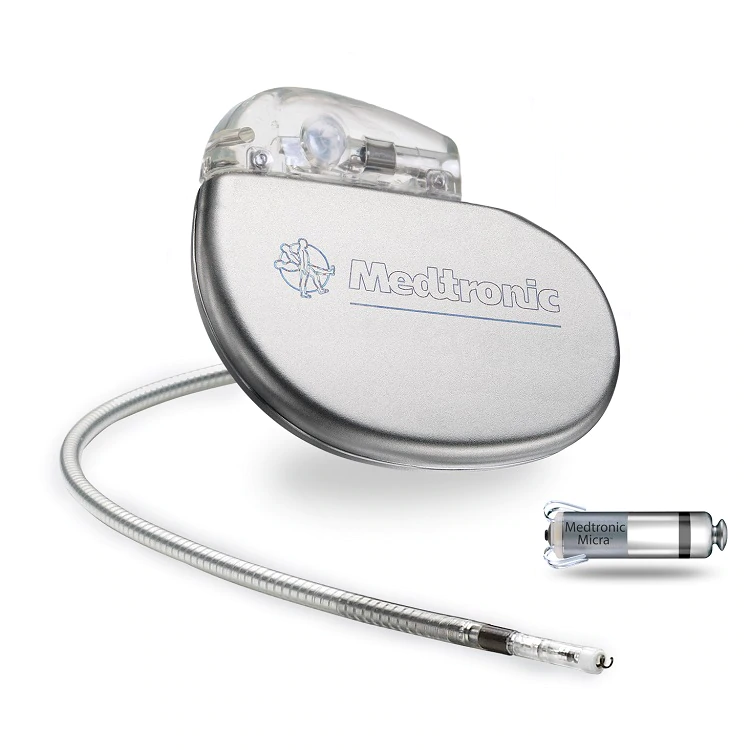
FDA Approves The World's Smallest Pacemaker
Revolutionary new transcatheter pacemaker is implanted inside the heart.
Perhaps more than most people, Mary Lou Trejo appreciates history.
A retired librarian, at age 79 she’s putting together a family history for her grandchildren, one that includes more than its share of tragedy.
“My grandkids aren’t going to know if I don’t do this,” she said.
A heart attack claimed her grandfather at 54, before any of his grandchildren were born.
Her father Ray, tall and thin all his life, also died of a sudden heart attack, at just 59.
Mary Lou was just 25 years old at the time. She vividly remembers the moment.
“Very scary. I heard him collapse. I heard a rattle. He was gone. Just like that,” she said.
Despite living healthy and staying active, Mary Lou began having her own heart problems around the same age.
Years of drug therapy helped, but eventually the doctor said she needed a pacemaker.
“He said I had two options,” she recalls. “I could get the traditional pacemaker or this new one.“
About the size of one of Mary Lou’s calcium pills, “this new one” was the Medtronic Micra, the world’s smallest pacemaker. In clinical trials at the time of Mary Lou’s procedure, Micra was approved for use in the United States on April 6, 2016.
Revolutionary not only for its size, Micra is implanted inside the heart. Small tines then attach it to the heart wall.
Doctors guide it from the groin directly into the heart through the femoral vein.
Dr. Ralph Augostini is the cardiologist who performed Mary Lou’s procedure. He says another major advantage of the new technology is eliminating the wires, or “leads” that connect traditional pacemakers to the heart.
“I think the leadless pacemaker is going to make a major impact in terms of reducing device-related infections,” he said. “I’m hopeful that will be the case.”
A study of clinical trial results, published in the New England Journal of Medicine, found that compared to traditional pacing systems, leadless pacing with Micra had significantly fewer major complications, like systemic infection, as well as the number of hospital stays.1
Micra is the result of a ten year program at Medtronic called “deep miniaturization.”
The goal a decade ago was to shrink medical devices by up to 90 percent, a goal achieved with the Micra.
Medtronic scientists, engineers and designers succeeded, by figuring out how to make a new generation of tiny devices that use only a fraction of the energy they once did.
Micra is among the first of those new devices to reach patients.
Dr. John Hummel is a cardiologist who participated in the clinical trials of Micra. He believes pacemaker therapy will be forever changed by wireless pacing technology.
“We are looking at the beginning of the future,” he said. ”We will no longer pace the heart in the way we have in the last 20 to 30 years. This is fundamentally a paradigm shift in how we’ll deliver this therapy,” he said.
As one of the first people in the United States to receive a Micra pacemaker, Mary Lou participated in clinical trials for the device. Now two birthdays removed from her procedure, she’s proud to have been a pioneer.
“Because there are just so many people that have heart problems,” she said. “And to be able to do something that advances how they are treated is just a nice feeling.”
Not to mention, adding another notable chapter in her family’s history.
IMPORTANT SAFETY INFORMATION: MICRA TRANSCATHETER PACING SYSTEM VVIR SINGLE CHAMBER WITH SURESCAN MRI
Indications
Micra Model MC1VR01 is indicated for patients with:
- symptomatic paroxysmal or permanent high grade AV block in the presence of AF,
- symptomatic paroxysmal or permanent high grade AV block in the absence of AF, as an alternative to dual chamber pacing when atrial lead placement is considered difficult, high risk, or not deemed necessary for effective therapy,
- symptomatic bradycardia-tachycardia syndrome or sinus node dysfunction (sinus bradycardia/sinus pauses), as an alternative to atrial or dual chamber pacing when atrial lead placement is considered difficult, high risk, or not deemed necessary for effective therapy.
Rate-responsive pacing is indicated to provide increased heart rate appropriate to increasing levels of activity.
Contraindications
Micra model MC1VR01 is contraindicated for patients who have the following types of medical devices implanted: an implanted device that would interfere with the implant of the Micra device in the judgment of the implanting physician, an implanted inferior vena cava filter, a mechanical tricuspid valve, or an implanted cardiac device providing active cardiac therapy that may interfere with the sensing performance of the Micra device.
The use of deactivated Micra devices in situ and an active Micra device, or an active transvenous pacemaker or defibrillator, has not been clinically tested to determine whether EMI or physical interaction is clinically significant. Bench testing supports that implantation of an active Micra device, or an active transvenous pacemaker or defibrillator, next to an inactivated Micra device is unlikely to cause EMI or physical interaction. post-approval studies are planned to characterize risks of co-implanted, deactivated Micra devices. Currently recommended end of device life care for a Micra device may include the addition of a replacement device with or without explantation of the Micra device, which should be turned off.
Contraindications
The device is contraindicated for patients who have the following conditions: femoral venous anatomy unable to accommodate a 7.8 mm (23 French) introducer sheath or implant on the right side of the heart (for example, due to obstructions or severe tortuosity), morbid obesity that prevents the implanted device from obtaining telemetry communication within ≤12.5 cm (4.9 in), or known intolerance to the materials listed in the Instruction for Use, or to heparin, or sensitivity to contrast media that cannot be adequately premedicated, or where a single dose of 1.0 mg dexamethasone acetate may be contraindicated.
Precautions should be taken before administering anticoagulant agents, antiplatelet agents, or contrast media in patients with known hypersensitivity to these agents.
The following may be contraindications to Micra VVIR pacing. Rate-responsive mode may be contraindicated for patients who cannot tolerate pacing rates above the programmed Lower Rate. Asynchronous VVIR pacing with sinus rhythm may be contraindicated when competitive pacing is considered undesirable or causes symptoms of pacemaker syndrome. The patient’s age and medical condition should be considered by physicians and patients as they select the pacing system, mode of operation, and implant technique best suited to the individual.
Warnings and Precautions
End of Service (EOS) – When the EOS condition is met, the clinician has the option of permanently programming the device to Off and leaving it in the heart, or retrieving the device, provided the device has not yet become encapsulated. Removal of the Micra device after it has become encapsulated may be difficult because of the development of fibrotic tissue. If removal of the device is required, it is recommended that the removal be performed by a clinician who has expertise in the removal of implanted leads.
MRI conditions for use – Before an MRI scan is performed on a patient implanted with the Micra device, the cardiology and radiology professionals involved in this procedure must understand the requirements specific to their tasks as defined in the device manuals.
Potential Complications
Potential complications include, but are not limited to, toxic/allergic reaction, oversensing, acceleration of tachycardia, myocardial infarction and surgical complications such as cardiac perforation, pericardial effusion, cardiac tamponade, death, device embolization, access site hematoma and AV fistulae, vessel spasm, infection, inflammation, and thrombosis.
See the device manuals for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, MRI conditions for use, and potential complications/adverse events. For further information, please call Medtronic at 1-800-328-2518 and/or consult Medtronic’s website at www.medtronic.com.
Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician.
REFERENCES
1 Micra had 54 percent fewer hospitalizations (p=0.011). Major complications included cardiac injuries (1.6 percent), complications at the groin site (0.7 percent) and pacing issues (0.3 percent).