Diabetes Therapy Earns Top Honors From Cleveland Clinic
In its annual list of up-and-coming technologies, the hospital gives the Medtronic MiniMed™670G the number one spot.
November 3, 2017 - Announced in late-October, Cleveland Clinic released its Top 10 Medical Innovations list. Each year, the hospital features what it calls “up-and-coming technologies with the potential to enhance healing and transform healthcare in 2018.”
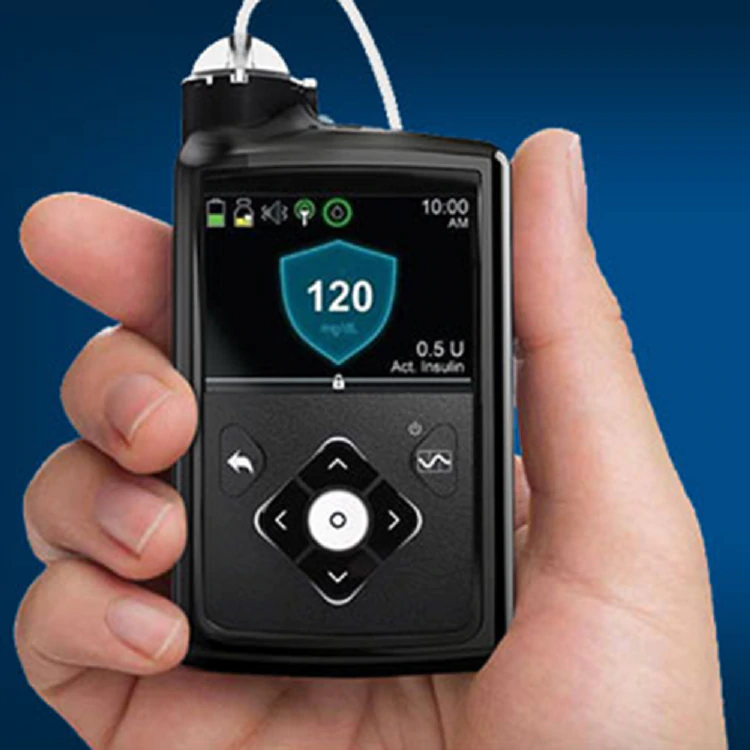
Topping the list is the Medtronic MiniMed™ 670G system, a hybrid closed-loop insulin delivery system — considered a breakthrough in diabetes therapy.
"I think it's a great number one pick that's going to have a dramatic impact on diabetes management in my opinion," said Dr. James Young, chairman of the Cleveland Clinic's Endocrinology and Metabolism Institute.
Approved by the FDA in 2016, the MiniMed 670G system helps make type 1 diabetes more manageable by enabling improved communication between the continuous glucose monitoring device and the insulin pump.
This year’s Top Medical Innovations list was announced at the Clinic’s annual Medical Innovation Summit. It was the 15th year of the list.
IMPORTANT SAFETY INFORMATION: MINIMED™ 670G SYSTEM
The Medtronic MiniMed 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, fourteen years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed 670G system includes SmartGuard technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitor sensor glucose values, and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian Sensor(3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian Sensor(3).Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site (palm) or from a control solution test. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
|
WARNING: Medtronic performed an evaluation of the MiniMed 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore, this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day, because the device requires a minimum of 8 units per day to operate safely. |
Only use rapid acting U100 insulin with this system. Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed 670G system has not been studied in pregnant women. For complete details, including product and important safety information concerning the system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information#minimed-670g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library