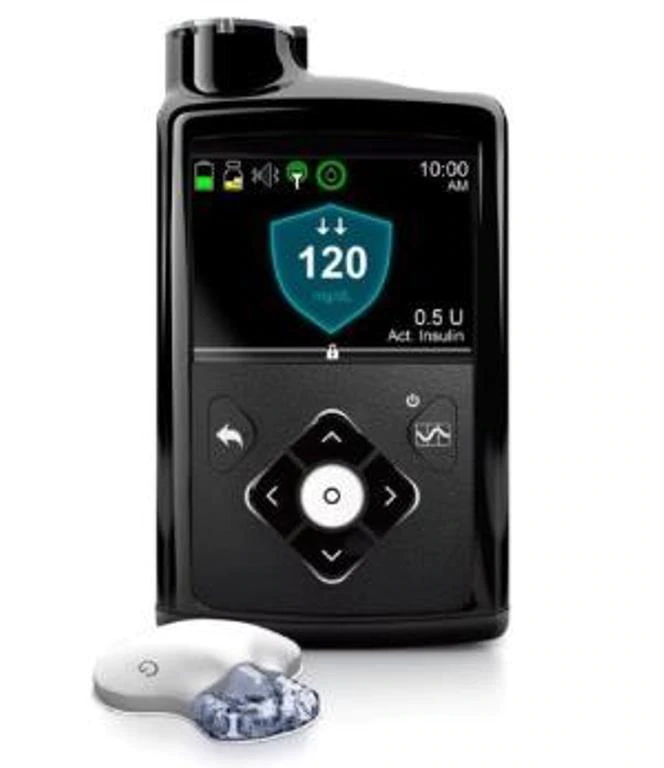
Medtronic Receives FDA Approval For ‘A Significant Milestone’ In Diabetes Care
The FDA approves world’s first hybrid closed loop system for people living with type 1 diabetes.
September 29, 2016 – When Tamar Sofer-Geri heard the news, the tears quickly followed.
“I was very excited,” she said. “I was surprised at how emotional I was. “
Tamar is not only the founder of a non-profit in California that provides awareness and support for people living with type 1 diabetes, but she’s also the mother of a teenager living with the disease.
That’s why the news meant so much.
“This is the biggest technological development since Tia was diagnosed,” says Tamar. “And to think that she had a part in making it happen makes me so proud.”
On Wednesday, Medtronic announced that it had received FDA approval for the world’s first hybrid closed loop (HCL) insulin delivery system – the MiniMed 670G system. It’s the company’s most advanced insulin pump and sensor system to date and provides patients living with diabetes more glucose control than ever before.
Tamar’s 16 year old daughter Tia was part of the MiniMed 670G system clinical trial. As an avid reader and soccer player, the high school junior knows that keeping her glucose numbers in range requires time and effort.
“With the HCL system, I’m spending less time managing my disease, and getting better results,” she says. “It adjusts to you and there’s just less you have to do.”
The system, which automatically adjusts the delivery of basal insulin, is also customizable so patients can choose from various levels of automation that best fit their management needs. The MiniMed 670G requires reduced patient interaction and uses the company’s most advanced SmartGuard™ HCL algorithm to minimize spikes and dangerous lows in blood sugar levels so patients feel better more often.
Dr. Richard M. Bergenstal, the principal investigator of the pivotal study and executive director of the Park Nicollet International Diabetes Center in Minneapolis says, “The ability to automate basal insulin dosing 24 hours a day is a much-anticipated advancement.”
Dr. Bergenstal believes the “therapy will be well-received by the clinical and patient community.” Others in the diabetes community are also praising the approval.
“This is a significant milestone,” says Derek Rapp, president and CEO of JDRF, the leading global organization funding type 1 diabetes research. “It’s an important step in the management of type 1 diabetes and will improve the quality of life for those living with this chronic disease.”
Gaining FDA approval for the first hybrid closed loop system took a strong commitment from Medtronic.
“It’s the culmination of years of hard work,” says Alejandro Galindo, president of Intensive Insulin Management for the Diabetes Group at Medtronic. “The speed by which the FDA approved our PMA application submitted in June is truly unprecedented – just 104 days from submission to approval. That speaks volumes about the potential impact this innovative therapy can have on patients in the diabetes community.”
Tamar calls the therapy “life-changing.”
“It’s remarkable how much I’m able to trust the system,” says Tamar. “Being able to send my daughter on an overnight trip without having to worry about her was something I couldn't have imagined doing a year ago. “
Medtronic says the innovating isn’t over.
“The system speaks to our commitment to simplifying and improving diabetes management through the advancement of smart algorithms that achieve greater glucose control with reduced patient input,” says Galindo. “This latest advancement brings us closer to our ultimate goal of developing a fully automated, closed loop system.”
“I feel huge relief knowing that in the very near future everyone will hopefully have access to this technology,” says Tamar. “It’s a game changer.”
The system is approved for treatment of people living with type 1 diabetes 14 years of age and older. A clinical study is ongoing to evaluate the safety and efficacy of the therapy in children ages seven to 13, as well as a feasibility study evaluating the therapy in children ages two to six.
Medtronic will begin commercial release in Spring 2017.
IMPORTANT SAFETY INFORMATION
The Medtronic MiniMed 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of Type 1 diabetes mellitus in persons, fourteen years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed 670G System includes SmartGuard HCL technology, which can be programmed to automatically adjust delivery of basal insulin based on Continuous Glucose Monitor sensor glucose values, and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed 670G System consists of the following devices: MiniMed 670G insulin pump, the Guardian Link (3) transmitter, the Guardian Sensor (3), One-Press Serter, and the Contour NEXT Link 2.4 glucose meter. The system requires a prescription.
The Guardian Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. All therapy adjustments should be based on measurements obtained using a home blood glucose monitor and not on values provided by the Guardian Sensor (3).
WARNING: Medtronic performed an evaluation of the 670G close loop system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore, this device should not be used in anyone under the age of 7 years old. This device should also not be used in patient who require less than a total daily insulin does of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
The MiniMed 670G system is not approved for use in pregnant women and patients with impaired kidney function. For complete warnings, precautions, and contraindications, please consult the User Guide.