Q2 earnings: three things to know
Product approvals and strength across businesses drive growth
Medtronic announced financial results for the second quarter of its 2024 fiscal year, reporting revenue growth and raising its guidance. Here are three things to know:
Delivering on innovation
Several key Medtronic products received regulatory approvals in recent months. During the second quarter, the U.S. Food and Drug Administration (FDA) approved the Aurora EV-ICD™ system. Unlike traditional implantable cardioverter defibrillators, Aurora is implanted below the left armpit and the lead is placed under the breastbone using a minimally invasive approach. The company also recently received approvals for the the Symplicity Spyral™ renal denervation (RDN) system in the U.S. and the PulseSelect™ pulsed field ablation (PFA) system in CE Mark countries.
Strong and broad fundamentals
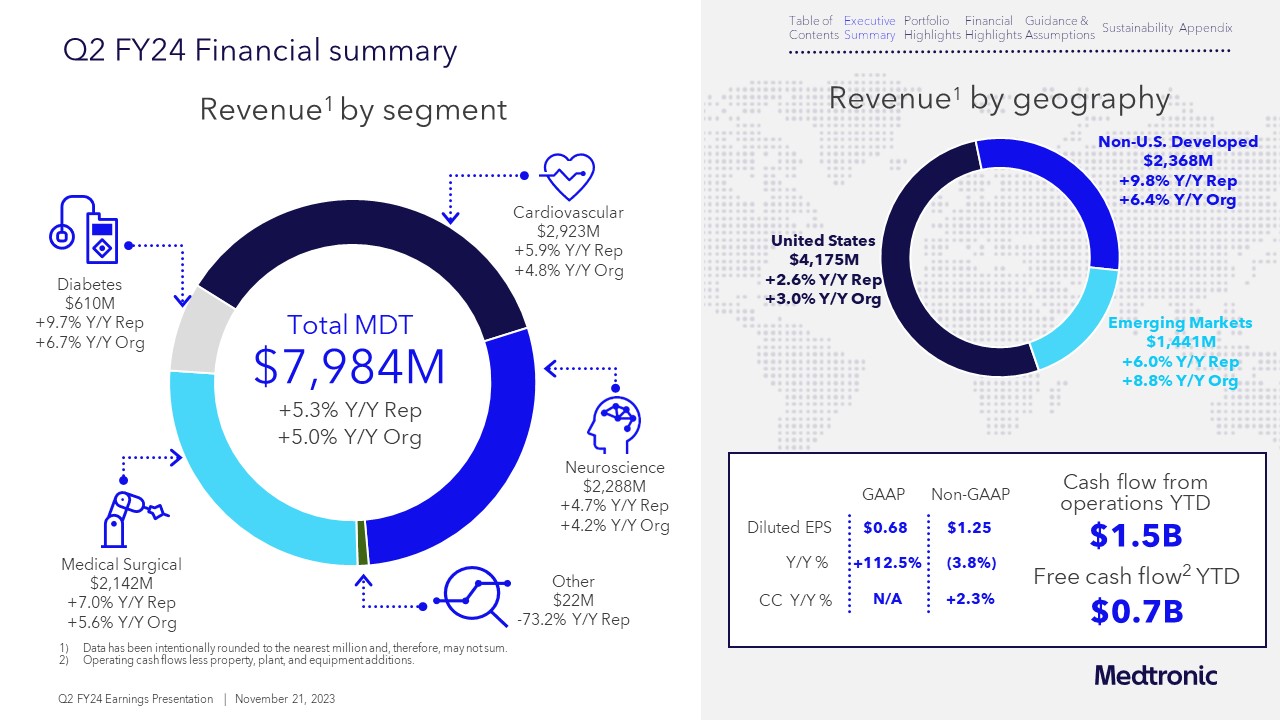
Medtronic reported growth across key businesses and continues to invest in fast-growing technologies such as artificial intelligence, robotics, and closed-loop systems. The company’s Cardiovascular, Neuroscience, and Medical Surgical business all increased revenue by mid-single digits and Diabetes achieved high-single digit growth.
Beating expectations , raising guidance
After outperforming expectations in the second quarter, Medtronic also raised its guidance – for both organic revenue growth and earnings per share – for full 2024 fiscal year.
L009-11202023
Related content