| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Dec 4, 2025
Now supported with effectiveness evidence, Medtronic DBS is the first and only U.S. FDA approved system to treat Dystonia symptoms
-
Sep 22, 2025
The pivotal Medtronic Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease (ADAPT-PD) trial demonstrates global clinical effectiveness, long-term safety, and patient preference
-
Jun 4, 2025
Minimally invasive laser technology now with enhanced capital system improvements, offering new user interface, workflow efficiencies, and upgrade capabilities
-
Jan 23, 2025
Study shows 67% mean reduction in Low-back Visual Analog Scale (VAS) pain score at 12 months; 87% of subjects had meaningful improvements in three or more quality of life (QoL) domains
Data to be presented at the North American Neuromodulation Society (NANS) Annual Meeting 2025; In all, Medtronic to highlight 22 poster and oral scientific data presentations
-
Sep 17, 2024
ADAPT-PD trial methods and preliminary data published in npj Parkinson’s disease
-
Aug 21, 2024
New MRI labeling extends active scan time for Medtronic DBS systems
-
Aug 12, 2024
First ever FDA approval provides more options to meet patients’ personalized needs, including those with Parkinson’s disease
-
Jun 20, 2024
Deal expands Pain Interventions portfolio, now offering both unipedicular and bipedicular vertebral augmentation solutions
-
Mar 1, 2024
Doubled probe count enables multi-level spine tumor ablations for increased flexibility and efficiency Medtronic plc, a global leader in healthcare technology, today announced that it has received...
-
Jan 18, 2024
86% of subjects preferred closed-loop SCS in a multicenter, randomized, crossover, single blind study of Medtronic’s investigational closed-loop SCS system at one month
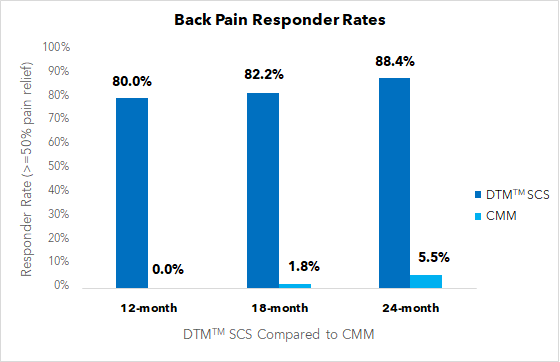
24-month European RCT data demonstrates long-term superiority of DTM™ SCS compared with conventional medical management (CMM) for indicated chronic back pain patients not eligible for spine surgery
-
Nov 10, 2023
NOVA Study: On-label, prospective, multicenter, randomized controlled trial compared DTM™ SCS to conventional spinal cord stimulation
-
May 17, 2022
Prospective study will evaluate the long-term performance of the closed-loop algorithm and overall patient experience with Medtronic’s next generation, rechargeable spinal cord stimulation device